| You are here: Home: BCU 7|2004: Patrick I Borgen, MD
Patrick I Borgen, MD |
EDITED COMMENTS |
 Clinical value of local control Clinical value of local control
I believe we’re on the precipice of a new appreciation for the value of local control in breast cancer. In the 1990s, the perception may have been that medical therapy could compensate for inadequate surgery or radiation therapy. However, recent studies, including the postmastectomy radiotherapy trials, have demonstrated that improved local control results in increased survival rates.
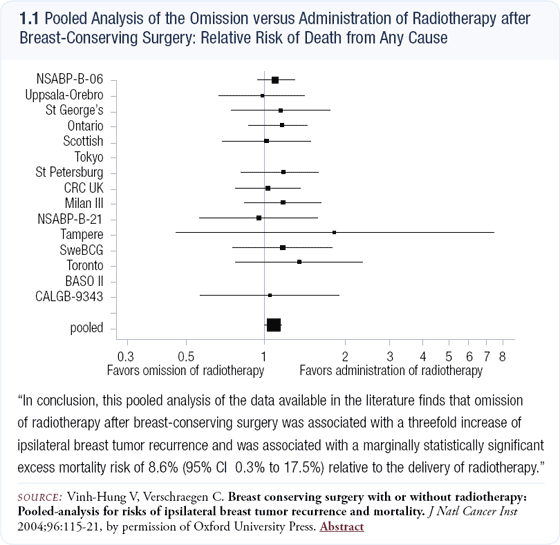
A meta-analysis published in the Journal of the National Cancer Institute evaluated virtually all of the lumpectomy and radiation therapy trials (1.1). Local control was defined by whether or not disease relapsed in the breast, and they specifically examined patients who received radiation therapy versus those who did not.
Whereas the NSABP-B-06 trial failed to show a survival disadvantage in the patients who experienced a local failure, when combined with all these studies and better follow-up, the importance of local control became very clear. The analysis demonstrated that patients with good local control had an eight percent better survival rate than those who experienced a local failure.
Impact of local failure
Studies in Milan and the United States, comparing mastectomy to lumpectomy and radiation therapy, demonstrated that the subset of patients who had positive nodes, received chemotherapy and were treated by breast-conserving therapy fared better than patients who underwent mastectomy. It has been postulated that a synergy exists between chemotherapy and radiation that we don’t understand. Nothing suggested the mastectomy group would do better in the future, and I don’t believe the long-term outcome of mastectomy will ever be superior to lumpectomy and radiation therapy.
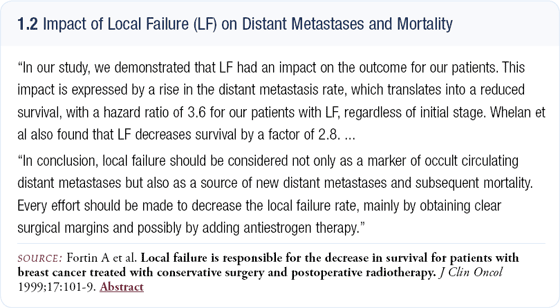
I don’t agree with those who contend that local recurrence is just a predictor of “bad biology.” A fascinating analysis from Canada by Dr Fortin and colleagues evaluated patients who had a breast cancer recurrence and patients who did not relapse (1.2). They found that all the patients had a certain risk of systemic disease, but the patients who had a local failure in the breast had a second risk of future systemic disease. They were able to demonstrate that as a time-dependent variable, local relapse was a cause rather than a marker of systemic relapse.
Partial breast irradiation
Conformal external beam radiation therapy is the most patient-friendly of the PBI techniques because it is noninvasive, quick and inexpensive. MammoSite® has generated a huge amount of enthusiasm, but it has limitations. A CT scan is necessary prior to treatment to ensure that the breast tissue abuts the device, and sometimes it doesn’t. Also, we teach our fellows that long-term cosmetic results are best when the disrupted tissues are put back together. It concerns me that with this procedure the surgical defect is not repaired. Brachytherapy — the technique with which we probably have the most experience — may prove to be a little too invasive for patients to accept.
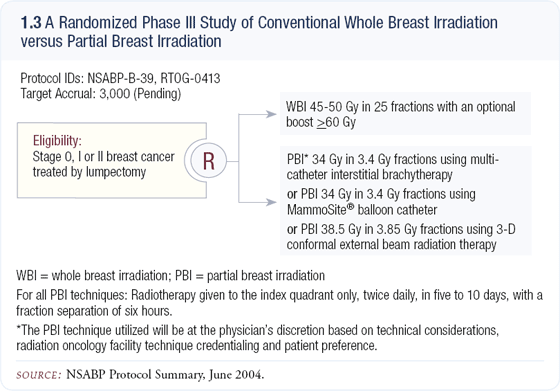
All of these PBI technologies lack large-scale prospective studies, so the NSABP is planning a trial in which the clinician can choose one of three different technologies: the brachytherapy technique of Kuske and colleagues, MammoSite® or conformal external beam partial breast radiation therapy (1.3). We are very enthusiastic about this study, and hopefully it will provide the data we need to truly evaluate PBI.
Ductal lavage
Leslie Montgomery from our group published a study in Cancer (Brogi 2003), in which ductal lavage (DL) was performed on 30 patients, 26 of whom had mammary carcinoma (1.4). The lavage samples were sent to three different pathologists, and none of them was read as cancer — not even one. I don’t believe DL should be compared to the Pap smear because it’s not an effective screening test, but it’s worth discussing as a risk assessment tool to identify atypical cells and select patients for chemoprevention. I believe its best use at this time is to retrieve cells from deep in the breast for intermediate biomarker research studies.
Current chemoprevention trials
The STAR trial has suffered from a lower-than-expected accrual due to the unpopularity of tamoxifen and the popularity of raloxifene. In addition, we have 40 years of experience with tamoxifen, and patients often have already decided which drug they want, which makes randomization difficult. These two agents are more alike than different and if raloxifene proves to be as effective as tamoxifen in prevention, it will be more readily accepted.
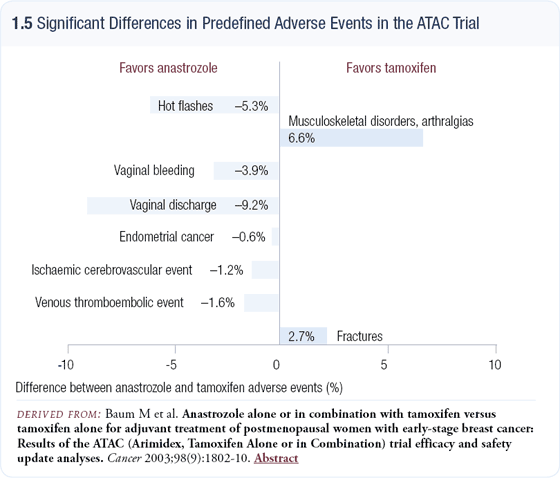
The IBIS-II chemoprevention trial comparing anastrozole versus placebo is even more exciting. In our experience with large numbers of patients, aromatase inhibitors are better tolerated than tamoxifen (1.5). Despite the results of the randomized trials, patients complain of weight gain on tamoxifen. Other problems include hot flashes, menopausal symptoms and possibly a low level of clinical depression.
Patients also worry about endometrial cancer and blood clots. With aromatase inhibitors, some arthralgias are reported, but these agents are very well tolerated. Convincing postmenopausal women at high risk to take an aromatase inhibitor rather than tamoxifen for chemoprevention will be an easier task if the trials demonstrate benefit.
Clinical trials of aromatase inhibitors in DCIS
NSABP-B-35 and IBIS-II are important trials, both comparing anastrozole and tamoxifen in postmenopausal patients with DCIS (1.6). Aromatase inhibitors have already been proven to have a significant effect in invasive cancer, and it’s highly likely they will impact DCIS as well. We know that the majority of DCIS lesions are likely to be ER-positive. Craig Allred has shown that age-per-age, tumor-for-tumor, DCIS is even more likely to be ER-positive than invasive cancer.
If that’s true, then we have even more reason to be optimistic about the studies of aromatase inhibitors in DCIS.
Clinical status of sentinel lymph node biopsy
It’s irrefutable that a sentinel node exists. Eighty studies around the world with over 10,000 patients — all with backup dissections and performed with a variety of techniques: methods, dyes and tracers — had the same results. I believe that the breast has a sentinel node, but I don’t believe it’s geographically specific or that we have to inject the dye close to the tumor. The challenge is to reliably find the sentinel node and recognize when the technique has failed.
Sentinel lymph node biopsy (SLNB) has moved to “prime time” faster than any other surgical approach for breast cancer. It took 80 years to advance from radical to modified radical mastectomy and 20 years to then adopt breast-conservation therapy.
It’s only taken approximately six years to move from axillary dissection to SLNB. With a relatively small amount of experience and coordination between nuclear medicine, surgery and pathology, SLNB is absolutely appropriate in the community setting.
SLNB in patients with DCIS
The indications for SLNB are still evolving. The easy answer to the question as to whether we should perform this procedure in patients with DCIS is, “No.”
Approximately 30,000 cases of DCIS occur annually in the United States. If we performed SLNB on slightly more than half, say 17,000 patients, with a positivity rate of approximately seven percent, which is what’s reported, 1,200 would have node-positive disease. Treating those 1,200 with chemotherapy would save approximately 61 patients, and that’s a high price to reduce mortality by 61 lives.
DCIS is the most rapidly growing subset of our breast cancer population. Not every case is pure DCIS, however, and the challenge for the surgeon is to identify the DCIS cases with invasion. We find that approximately 10 to 15 percent of our DCIS cases have a hint of invasion, such as architectural distortion on a mammogram or a palpable mass, so we perform SLNB on those cases and approximately 10 percent are positive.
We are conducting an exciting multi-institutional study, along with Mel Silverstein, examining a large number of patients with DCIS who underwent SLNB, and we’re following those cases longitudinally.
I do believe that all patients with DCIS who require a mastectomy should undergo SLNB. When we performed mastectomies in the past, we almost always removed two to four lymph nodes from the axillary tail. SLNB probably allows us to remove fewer nodes.
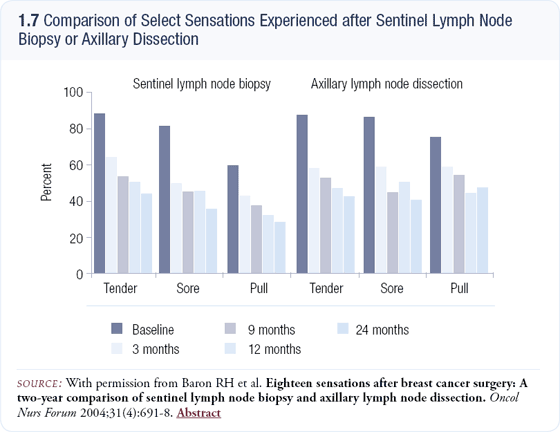
Neurosensory sequelae of SLNB
Roberta Baron from our group has conducted a study comparing the neurosensory morbidity of SLNB versus axillary node dissection (1.7). Surgeons have billed SLNB as relatively free of side effects, but Ms Baron’s study demonstrated that, although the intensity of symptoms was less following SLNB, the number of complaints about sensory morbidity in this study include pain, a pulling sensation, achiness and tenderness — which was the same after SLNB and postaxillary node dissection. The symptoms may be present for two or more years.
Select publications
 |
Dr Borgen is Professor of Surgery at Weill Medical College of Cornell University and Chief, Breast Service, Department of Surgery at Memorial Sloan-Kettering Cancer Center in New York, New York. |
|
