| You are here: Home: BCU 7|2004: Adam M Brufsky, MD, PhD
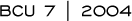
Adam M Brufsky, MD, PhD |
EDITED COMMENTS |
 Duration of trastuzumab in the metastatic setting Duration of trastuzumab in the metastatic setting
The duration of trastuzumab in metastatic disease has not been studied in a randomized trial, so we are conducting an observational study of 400 patients in approximately 50 centers, and every three months we’re recording each patient’s treatment. I expect we’ll find that about 35 percent of clinicians don’t continue trastuzumab after progression. Many believe that progression with a chemotherapy- trastuzumab regimen indicates resistance to trastuzumab, but I don’t agree.
I believe it is beneficial to continue trastuzumab beyond an initial progression, but I don’t know for how many progressions it continues to be advantageous. In our retrospective analysis of approximately 200 patients who received frontline trastuzumab, those who continued on trastuzumab seemed to have a small benefit, at least in time to progression, compared to those who did not. A retrospective study from the Hellenic Cooperative Oncology Group (Fountzilas 2003) demonstrated time to progression intervals of three to four months with thirdand fourth-line trastuzumab plus chemotherapy.
Cardiac effects in adjuvant trastuzumab trials
NSABP-B-31, which randomly assigns patients to AC followed by paclitaxel with or without trastuzumab, evaluated cardiac safety in the first 1,000 patients. The cardiac endpoint was the absolute difference in protocol-defined cardiac events between the two arms, and if it exceeded four percent, accrual would be terminated.
The cardiac event rates were 0.78 and 4.28 percent in the control and trastuzumab arms, respectively, so the study continued and for the vast majority of patients the cardiotoxicity was reversible. Still, the rate in the study arm equates to approximately one in 20 or 25 women, and that concerns me. When I counsel patients, I tell them about trastuzumab’s performance in the metastatic setting and that we’re excited about its potential in the adjuvant setting, but that it’s still unproven.
Trastuzumab-chemotherapy regimens for HER2-positive disease
The BCIRG conducted a Phase II study of docetaxel/cisplatin/trastuzumab in patients with HER2-positive advanced breast cancer and reported dramatic results in terms of time to progression (4.1). At approximately the same time, we began a 40-patient, Phase II trial of docetaxel/carboplatin/trastuzumab and we’re seeing similar results. Our response rate is between 70 and 80 percent, and time to progression is approximately 12 to 13 months.
The BCIRG-006 adjuvant trial compared adjuvant AC plus docetaxel with or without trastuzumab versus docetaxel/trastuzumab with either carboplatin or cisplatin in women with node-positive or high-risk, node-negative, HER2- positive, operable breast cancer. I expect the data from the docetaxel/carboplatin/ trastuzumab arm will be at least as good as that seen in the Phase II studies with regard to disease-free and overall survival, but with less cardiotoxicity. If that’s the case, then that regimen will become the treatment of choice for patients with node-positive, HER2-positive breast cancer.
HER2 testing
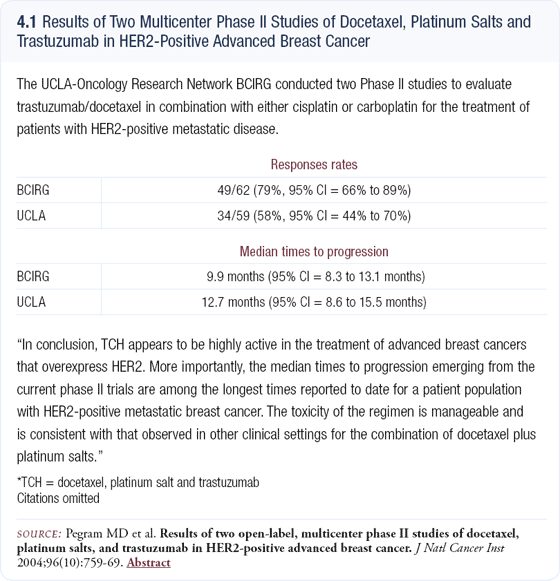
We initially perform IHC for HER2 testing and then FISH if the IHC result is 2+. We view zero and 1+ results as HER2-negative and 3+ results as HER2-positive. However, we know from concordance data that approximately 10 percent of zero and 1+ cases will be FISH-positive and approximately 10 percent of 3+ cases will be FISH-negative, so that has to be taken into consideration.
We have learned that labs must perform a high volume of FISH testing to be proficient, and community labs have low concordance rates. At the 2004 ASCO meeting, an interesting technique for evaluating the HER2 status was presented, called chromogen in situ hybridization (CISH). The concordance rates between this technique and FISH were high, and I believe this new assay will change our current patterns of testing (4.2).
First-line therapy for women with HER2-positive metastatic disease
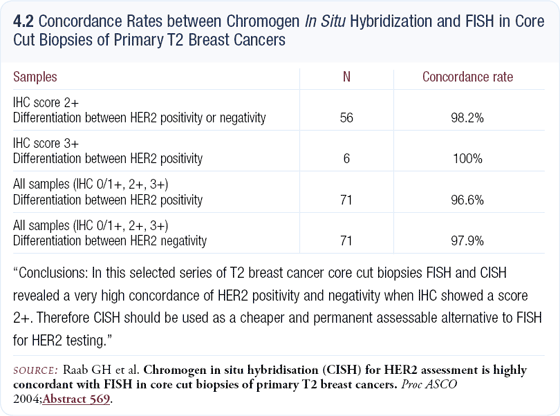
In selecting first-line therapy for patients with HER2-positive metastatic disease, I consider the pace of the disease and the patient’s desires. If a patient can tolerate chemotherapy and has substantial disease in the liver or lungs, I use docetaxel/ carboplatin/trastuzumab. In an older woman or a frail patient or a woman who doesn’t want to lose her hair, I select vinorelbine/trastuzumab. If the patient has ER- and PR-negative disease with only bone or maybe a few soft-tissue metastases, I use trastuzumab alone. In Vogel’s data, approximately 25 to 35 percent of women with metastatic, FISH-positive disease responded to single-agent trastuzumab (4.3).
I’ve also used a combination of capecitabine and trastuzumab in the first-line metastatic setting in select cases. For example, in patients with very high bilirubin levels, I find it difficult to give a taxane or anthracycline. However, an abstract presented at ASCO several years ago showed it was safe to use lower-dose capecitabine in these patients. In vitro data from Slamon and Pegram showed that perhaps these drugs were additive and many clinicians, I believe, overinterpreted that data and felt capecitabine shouldn’t be combined with trastuzumab. I don’t necessarily agree and a number of clinicians, including myself, have had some success with this combination.
Adjuvant chemotherapy trials
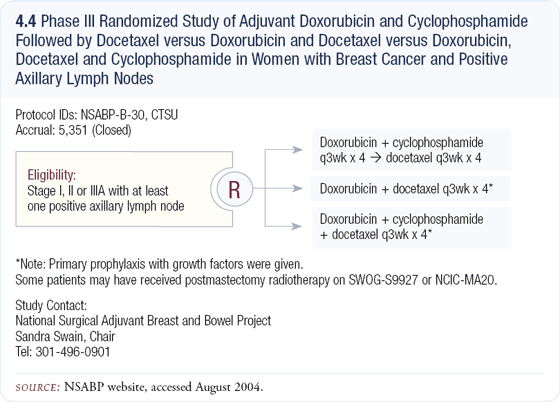
Many of us are concerned that two of the three regimens are suboptimal in the NSABP-B-30 three-arm, Phase III adjuvant chemotherapy trial (4.4). In this study, patients with node-positive disease are randomly assigned to doxorubicin/cyclophosphamide (AC) followed by docetaxel (T) or doxorubicin/docetaxel (AT) or TAC. The TAC regimen is only four cycles rather than six, which I believe is too little chemotherapy. I also believe the AT arm is likely to be inferior to AC followed by docetaxel.
When you look at the neoadjuvant data, the pathologic complete response rate following four cycles of AT was approximately seven percent versus 11 to 12 percent for AC followed by docetaxel. I believe pathologic complete response rates will likely translate to better disease-free and overall survival.
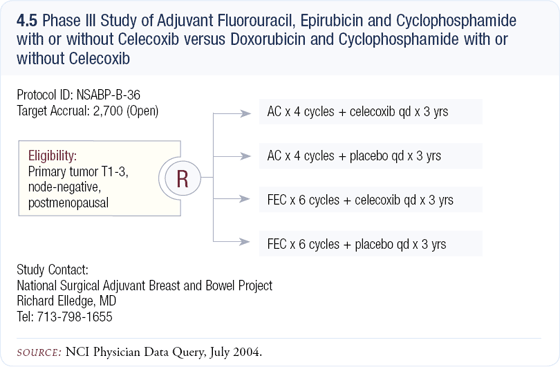
I’m very interested in the NSABP-B-36 study in patients with node-negative disease, which compares AC versus fluorouracil/epirubicin/cyclophosphamide, and then all patients are randomly assigned to celecoxib or a placebo (4.5). They are using the non-dose-dense AC in this trial, which I support. I believe the data from CALGB-9741 favoring dose density was related to the dose-dense administration of paclitaxel rather than AC.
Fulvestrant in the adjuvant and metastatic settings
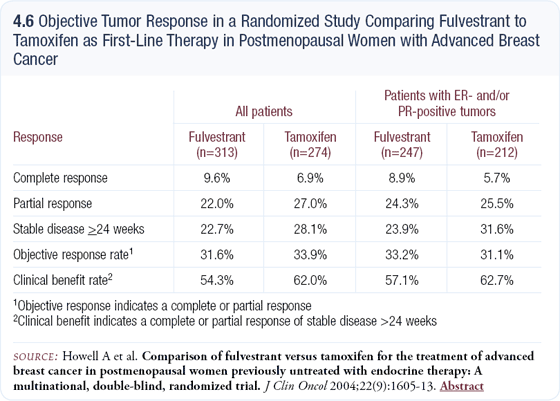
Most clinicians consider fulvestrant a third-line therapy for patients who have failed tamoxifen and an aromatase inhibitor; however, clinical trials have shown fulvestrant is equivalent to anastrozole after tamoxifen failure and, in a recently published European study comparing front-line fulvestrant versus tamoxifen, I did not view tamoxifen as inferior (4.6).
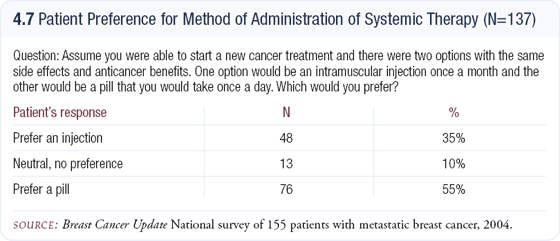
In addition, a Phase III study is underway comparing fulvestrant to exemestane for second-line therapy. I do use third-line fulvestrant, but I will use it first-line, particularly in women who can’t afford an aromatase inhibitor. In addition, I would estimate that approximately 40 percent of my patients prefer a monthly injection to taking a pill every day (4.7).
I believe aromatase inhibitors will be difficult to beat, but many of us are interested in adjuvant studies with fulvestrant. It has a lot of advantages and I would like to see it compared to aromatase inhibitors in postmenopausal women in the adjuvant setting.
Adjuvant therapy in postmenopausal women with ER-positive tumors
Off protocol in a postmenopausal woman, I generally use adjuvant anastrozole up front or, if the patient has been on tamoxifen for two or three years, I switch her to exemestane. After five years of tamoxifen therapy, I offer patients letrozole. The issue here is that because patients generally do well after five years of tamoxifen, we have to carefully weigh the potential benefit and side effects of further adjuvant therapy. A patient with a small tumor may not need it; however, in a patient with multiple positive nodes, it probably is indicated.
In women who have been off adjuvant tamoxifen for a while, my cut-off to start an aromatase inhibitor is approximately one year, although it’s probably acceptable to start at any time. At least in preventing new breast cancer, although further therapy is likely advantageous, but again one has to balance side effects with benefit. Even though we know there’s still a linear rate of recurrence after five years of tamoxifen, if the disease hasn’t recurred two or three years later, I believe that tells me something about the biology of that patient’s disease.
Select publications
 |
Dr Brufsky is an Assistant Professor of Medicine at the University of Pittsburgh, Member of the University of Pittsburgh Cancer Institute, Director of the Comprehensive Breast Cancer Center, and Associate Division Chief of the Department of Medicine’s Division of Hematology/Oncology at the University of Pittsburgh in Pittsburgh, Pennsylvania. |
|
