| You are here: Home: BCU 5|2004: Hyman B Muss, MD
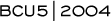
Hyman B Muss, MD |
EDITED COMMENTS |
 Intergroup trial of adjuvant capecitabine in elderly women Intergroup trial of adjuvant capecitabine in elderly women
CALGB-49907, which is an Intergroup trial also available through the Cancer Trials Support Unit (CTSU) of the NCI, compares capecitabine, an oral 5-FU prodrug, with CA or CMF.
In this trial, for patients randomly assigned to standard therapy (CA or CMF), the physician chooses which of these two regimens to use. The goal is to determine whether capecitabine is equally effective as standard adjuvant therapy.
The women eligible for this trial are 65 years and older with node-positive or high-risk, node-negative breast cancer. Women with ER-positive tumors can receive tamoxifen or anastrozole as their endocrine therapy. So far, the trial is accruing reasonably well.
We are gathering excellent quality-of-life data and collecting adherence data with an electronic pill bottle. We are also evaluating some incredible laboratory science including genes that might tell us about toxicity, such as levels of thymidine phosphorylase, thymidylate synthase and dihydropyrimidine dehydrogenase (DPD). In addition, we’ll be storing all the blocks for future work.
We’re excited about this study. While it’s a little early for me to predict how to compare these regimens, I believe patients may perceive that capecitabine is a little easier to take because it is oral and not associated with alopecia.
Dosing of capecitabine and monitoring for toxicity
In CALGB-49907, capecitabine is given at a dose of 2,000 mg/m2 per day in divided doses for 14 consecutive days every three weeks for six cycles. We initially escalated the dose to 2,500 mg/m2, but we elected to reduce it because of severe toxicity. I believe this lower dose is certainly adequate. Rarely can patients tolerate a full dose on a continuous basis as indicated in the package insert.
Two patients out of the first 60 in the trial died, probably as a result of capecitabine toxicity. The first patient had profound DPD deficiency, and within several days of starting capecitabine she developed severe diarrhea and mucositis and was admitted with marked pancytopenia. She became septic and died.
The second patient had some modest diarrhea after her sixth course at 2,500 mg/m2, and unfortunately the clinicians caring for her could not convince her to come into the clinic when she called. Over a period of weeks she became more symptomatic, developed a severe infection and died.
The incidence of DPD deficiency is probably less than one percent. Unfortunately, no reliable test exists to screen for this disease. It is not practical to measure DPD because large quantities of blood are needed. The deficiency is likely a polymorphism in a protein that is present but not functional.
Based on our experience with the DPD-deficient patient, we amended the protocol to identify these patients. We now have women come in between days four and six of the first cycle and again several days later for “mini checks.” We do this to make sure we don’t miss patients who may have profound toxicity early. These checks will enable us to stop the drug early and avoid serious toxicity.
Our assessment is that capecitabine is a reasonably safe drug, but patients need to be informed. Doctors who don’t frequently use capecitabine need to be aware of this early toxicity, and older patients should be contacted and assessed.
Use of capecitabine in the metastatic setting
I treat a majority of patients, old and young, with capecitabine as first-line therapy in the metastatic setting. I find that many patients, especially the elderly, are wary of chemotherapy. Despite all of our wonderful endocrine agents, at some point most women progress and need chemotherapy. Capecitabine is a nice way to initiate a patient into treatment — it is oral, doesn’t cause hair loss and is a gentle way to begin chemotherapy. In addition, the response rates are comparable to the response rates of taxanes, gemcitabine and vinorelbine.
I pay close attention to hand-foot symptoms because that can be a difficult problem — especially in the elderly patient. I usually start with a dose somewhat lower than 2,000 mg/m2, especially with older patients, and then raise it quickly rather than risk that a patient will develop toxicity and decide not to receive any more chemotherapy. Overall, I believe capecitabine is an excellent drug.
Single agents versus combination chemotherapy in the metastatic setting
Based on the best published data, I believe that single-agent sequential therapy is still the best way to manage most patients with metastatic breast cancer. It is less toxic, you’re not lowering dose — and perhaps efficacy — below a threshold level, and survival is identical. Additionally, other drugs can be offered later. I believe single-agent sequential therapy is the way to go, and I start with capecitabine first in most patients.
I don’t believe vast differences exist with regard to responses and confidence intervals; however, there are exceptions, such as the patient with terrible bone pain or in whom another doubling of their liver or pulmonary metastases will be catastrophic. Occasionally patients have pleural effusions that are difficult to manage. While achieving a faster response is helpful in these cases, these are the minority of patients.
When I use combinations, I use agents like capecitabine and docetaxel (2.1). In chemotherapy-naïve patients, anthracyclines and taxanes have very high response rates, but in the last several years in my practice I have started with a combination regimen in only about 10 percent of patients.
Breast cancer is not high-grade lymphoma or acute myeloid leukemia. We have time to work with the patients. This is a really tough problem for which all of our therapy is palliative.
New strategies for adjuvant and neoadjuvant clinical trials
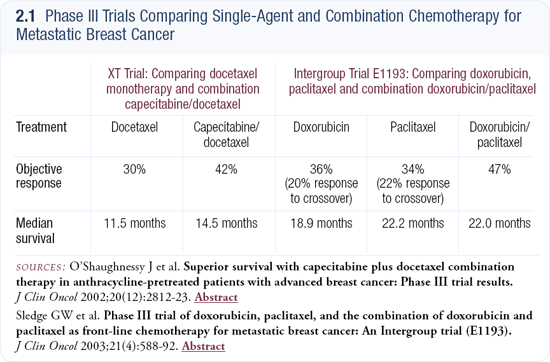
I believe the adjuvant trials studying the combination of capecitabine and docetaxel are wonderful trials to evaluate extremely active drugs in the adjuvant setting (2.2). We have several outstanding agents with high response rates in the metastatic setting, such as capecitabine, vinorelbine and gemcitabine, which haven’t been evaluated in the adjuvant setting. I support the strategy of moving these agents into the adjuvant course of treatment.
The neoadjuvant setting is another arena in which I believe we need more research (2.3). It is not uncommon for us to see patients after preoperative therapy and surgery who have seven positive nodes and scattered tumor throughout the breast. We don’t know what to do in these cases. Obviously we put patients with ER- or PR-positive tumors on endocrine therapy, but I don’t think any of us believes this is going to be a great strategy. I believe exploring agents such as capecitabine in those patients is a great idea.
I also think that some breast cancers have very few cells in cycle kinetically — like low-grade lymphomas. We will never cure these patients with aggressive agents, but perhaps metronomic, low-dose therapy — whether it’s weekly taxanes, weekly anthracyclines or capecitabine for a prolonged period of time — would treat that component of cells that aren’t cycling. All of these are great options for future studies.

Potential for use of vinorelbine/capecitabine (VINOCAP) combinations
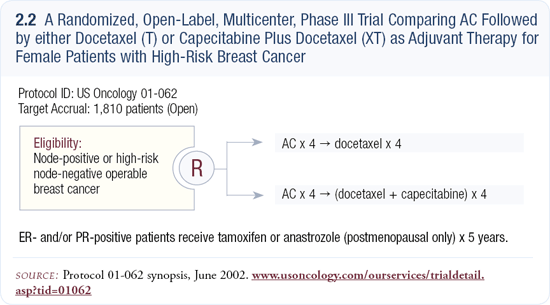
Some excellent Phase I and II abstracts have shown good response rates and modest toxicity with VINOCAP (2.4). I have occasionally used this in patients with metastatic disease who have progressed quickly. This is an example of a combination that would be great to study in large numbers in the adjuvant setting — for example, AC or TAC up front followed by VINOCAP. I believe these are extremely effective combinations when compared to some of the biologic agents, which we’re trying to move up front despite having very little efficacy data.
Nonprotocol adjuvant management of patients with positive nodes
Right now, I believe that TAC and dose-dense AC followed by T are among the two best choices for adjuvant chemotherapy in node-positive patients. I use more dose-dense therapy, and by limiting anthracyclines to four courses, perhaps we will have somewhat less cardiotoxicity in the long run. I’ve occasionally observed cardiotoxicity with some of the six-cycle or more anthracycline regimens. This is more of a gut feeling than a scientific observation, and I believe both regimens are excellent.
In terms of quality of life and toxicity, my interpretation is the regimens are not drastically different. You must use growth factors with TAC because the rate of neutropenic fever can be ameliorated with filgrastim or preferably pegfilgrastim.
Surprisingly, patients stay on cycle with the use of growth factors. My experience is that when using growth factors, very few people are neutropenic at the twoweek point. Any experienced clinician knows that at some point, with six cycles every three weeks of any therapy without growth factors, a delay will occur. My experience using growth factors with dose-dense therapy or TAC is that you will almost always be there on day 14 or day 21, ready to treat the patient.
Dose reduction and delay
I rarely use non-dose-dense therapy; however, for physicians who do, and have neutropenic patients on the day of planned therapy, I lean towards the use of growth factors rather than dose reduction. A threshold dose probably exists for anthracyclines, and I suspect growth factors are a way of making sure you hit that threshold.
I believe that some physicians “low-ball” patients on the dose of therapy in trying to be “nice” and minimize toxicity. However, if you start at half the dose because you believe the patient is fragile, you’re doing the patient a disservice. I think you need to evaluate the data and treat patients according to how they were managed in the protocol.
I think people are becoming more aware that a threshold effect probably occurs — the word gets out. I also believe that growth factors allow people to stay on schedule because you don’t see the profound drops in counts and the high rates of neutropenic fever. Hopefully this will translate to better efficacy outcomes in adjuvant therapy in the future.
This also may explain why postmenopausal patients in the Overview appeared to receive half the benefit from chemotherapy that younger patients received. Biologically, I can’t understand why that happens, yet it seems consistent over 15 years in the Overview. I suspect a large part may be dosing issues in the older studies that dominate the Overview. Perhaps this will change in future analyses.
Dose-dense therapy or TAC in patients with negative nodes
I believe that using dose-dense therapy or TAC is not outrageous in a very high-risk, node-negative patient. Using one of the prognostic models available, like Peter Ravdin’s ADJUVANT! program, some node-negative patients have worse outcomes than node-positive patients. For example, someone with a small ER/ PR-positive, low-grade tumor with one positive node has a better prognosis than someone with a 3.5-centimeter, receptor-negative, high-grade tumor with lymphovascular invasion and negative nodes.
I believe it is reasonable to use dose-dense therapy in node-negative patients at high risk. I’ve done this on occasion, because by using a program like ADJUVANT!, it is clear that the benefit can be very substantial in going from an AC or CMF regimen to a FEC regimen, a TAC regimen or a dose-dense regimen.
Use of fulvestrant in clinical practice
I’ve used fulvestrant, and my experiences have been good. It’s an excellent drug, and we’ve seen some good responses. Fulvestrant provides an excellent option for patients with slowly progressive metastases, irrespective of site. I use it more in the third-line setting after an aromatase inhibitor in women who progress after adjuvant tamoxifen.
Patients come in every four weeks for an injection. Many of these women are also receiving bisphosphonates, so they come in and we give them their bisphosphonate and their fulvestrant injection and check them over. Fulvestrant is very well- tolerated and I can’t recall any major side effects. While some patients find the injection a little uncomfortable, most patients tolerate it well and do not get sick — nor do they have to remember to take pills. I haven’t found the injection to be a major issue.
Select publications
 |
Dr Muss is a Professor of Medicine at the University of Vermont and Director of Hematology/ Oncology at Fletcher Allen Health Care in Burlington, Vermont. |
|
