| You are here: Home: BCU 5|2004: Clifford A Hudis, MD
Clifford A Hudis, MD |
EDITED COMMENTS |
 Practical implications of the MA17 trial Practical implications of the MA17 trial
With patients just finishing five years of adjuvant tamoxifen, I always discuss the MA17 data for switching to letrozole, which has demonstrated improvement in disease-free survival.
We don’t know how long to give letrozole or what the long-term overall health implications will be. Nor do we know whether it will have an overall survival impact, although my bias is that it will. I was struck by how high the risk of recurrence was in the second five years in the MA17 data. The estimated recurrence rate was 13 percent in patients on placebo and seven percent in patients on letrozole. This risk of recurrence illustrates why an efficacious therapy after adjuvant tamoxifen is desirable. We don’t have subset data to guide us in treating patients with small, node-negative breast cancers. I concede that the predicted benefit for these patients would be small, but we need clinical trials examining these subsets.
Letrozole initiation after prior history of adjuvant tamoxifen
We don’t know how long after a patient completes five years of adjuvant tamoxifen it is still beneficial to initiate letrozole. I consider the NSABP-P-1 prevention trial and the patient’s risk for recurrence at that point. The P-1 trial showed that if we intervene, we change a woman’s hazard rate for breast cancer occurrence, but we don’t know at what point the reduction in hazard rate becomes so low it is of marginal value (1.1).
For a patient with a 1.1-centimeter, node-negative breast tumor, intervention might still be beneficial a couple years after finishing tamoxifen, but for a patient with eight positive nodes and a 2.5-centimeter tumor, I would be willing to treat her further out because her hazard rate is probably still relatively high. When the results of the MA17 trial were revealed, the patients on placebo were offered letrozole even though we didn’t know whether it would be effective two or three years after tamoxifen.

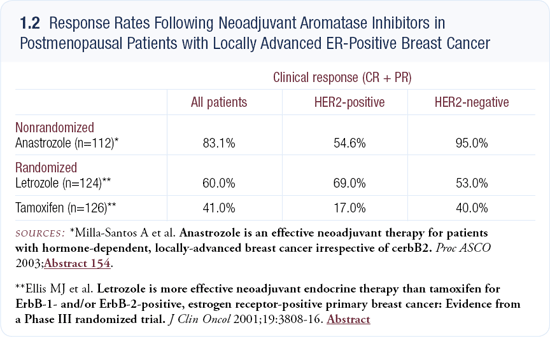
Aromatase inhibitors versus tamoxifen in HER2-positive tumors
Retrospective subset analyses of several trials show higher response rates with aromatase inhibitors than with tamoxifen in HER2-positive tumors. This area continues to evolve and I don’t believe we have the final answer, but the overarching trend seems clear (1.2).
CALGB-9741: Dose-dense chemotherapy
Unlike some other trials, analysis of CALGB-9741 was time-driven, not eventdriven. I’m glad we didn’t have an event trigger because we’d still be waiting for this important data, and results are only relevant for a certain period of time. The study stipulated an analysis at 36 months and, consistent with trends in adjuvant therapy in general and adjuvant therapy trials in particular, the actual number of events at 36 months was far less than expected — 315 events for event-free survival rather than the expected 515 events. The data revealed a statistically significant advantage to every two-week versus every three-week therapy but no difference between sequential versus concurrent AC.
My coauthors and I are confident of our report on CALGB-9741 because of the hazard rates for recurrence. I believe medical oncologists need to pay attention to the risk of recurrence each year. For node-positive breast cancer, the hazard function peaks early, at about two to three years, and then drops off. In this trial, at year four in follow-up we were already well down on the hazard function curve — a bell-shaped curve at the beginning.
The peak incidence of recurrence on a yearly basis had already passed, and seeing an advantage at this point told us that the every two-week therapy would always have an advantage in this trial. That won’t change because most of the events have already transpired.
Hazard rates of recurrences
Some criticize the data from CALGB-9741 because the magnitude of benefit over time may not be as large as it is now. That’s fair, because it could fluctuate, but the positivity won’t go away. We saw the same phenomenon in CALGB-9344 — if you plot the hazard function and compare paclitaxel to no paclitaxel, sometimes the curves are close together and sometimes the curves are further apart, but the aggregate benefit is clear and consistent.
Some physicians thought we got excited too early about the CALGB-9344 data, but I believe they focused on the raw numbers instead of the trend. Similarly, based on the 47-month follow-up of the ATAC trial, we can predict that the fiveyear disease-free survival will continue to be greater for patients on anastrozole, even though the magnitude may be different. Both ATAC and CALGB-9741 have enough patients and events to be statistically significant, and these hazard rates will not invert in years to come.
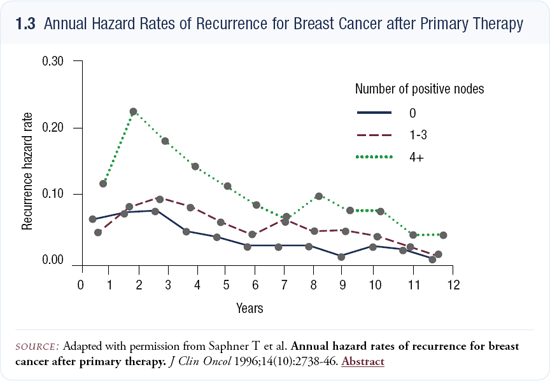
Natural history of recurrences and nodal status
The traditional view has been that node-positive patients have an early spike in recurrence, then after three to five years an inflection in their hazard rate occurs, and then it declines at a constant rate. For node-negative breast cancer, no early peak occurs — just a constant rate of recurrence.
I’m not certain this analysis would be so dichotomous with modern data sets and staging. I believe we need to explore this carefully. I can tell a patient with nodepositive disease who is disease-free at 36 months that her chances of recurrence are lower than any time earlier in her history, but that’s all I’m certain of. After five years without recurrence, patients are in a very good prognostic group, and many statisticians would argue that their original nodal status doesn’t matter a great deal, but that their risk of recurrence in the second five years is fairly constant (1.3).
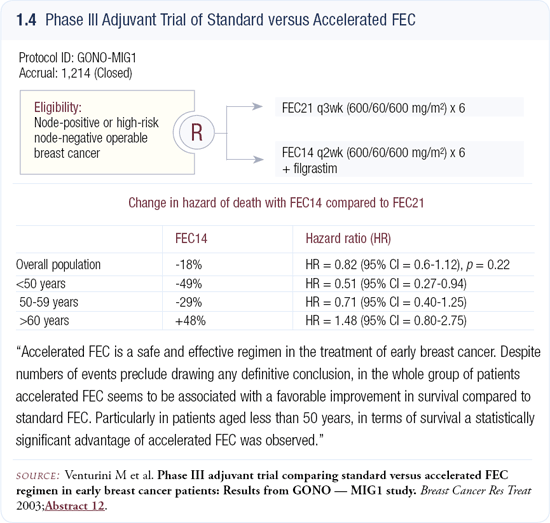
Dose-dense study of FEC
At the 2003 San Antonio Breast Cancer Symposium, Venturini et al presented data from a trial comparing FEC every two weeks versus every three weeks (1.4). It’s one of the few studies that, like CALGB-9741, truly tested dose density because every patient received the same doses of the same drugs for the same number of cycles and the only variable was the interval between treatments. I commend Venturini and his colleagues because that approach is the key to demonstrating the value of dose-dense therapy.
We hoped Venturini’s trial would confirm CALGB-9741 as a general principle, but their event rate was lower than expected and the study lost its power (1.4). In CALGB-9741, we also had fewer events than expected. Fortunately, our trial was large enough to demonstrate the benefit of dose density at 36 months. They presented the data showing a trend in favor of the dose-dense therapy, stating that while the trial was not positive, the range of possibilities included positivity.
Consistent with CALGB-9741, they were able to show that dose-dense therapy was faster with fewer episodes of febrile neutropenia. Although I was disappointed that their study didn’t have the power to confirm the CALGB data, I’m confident that their data was consistent with ours.
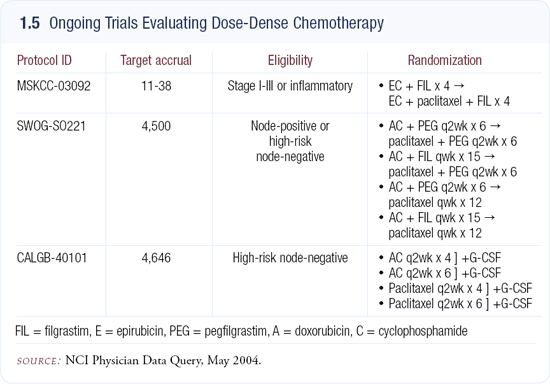
Integrating dose density into new clinical trials
Several trials are now incorporating the every two-week schedule (1.5). At my institution we’re evaluating even shorter intervals without compromising dose. In MSKCC-03092, patients receive epirubicin/cyclophosphamide (EC) at 10- or 11-day intervals for four cycles followed by four subsequent cycles of paclitaxel at 10- or 11-day intervals. I’m confident paclitaxel will be tolerable at that schedule, but the practical questions are whether we can use it immediately following EC and whether the anthracycline can be tolerated with such short intervals.
We’re also going to study six cycles of EC followed by six cycles of a taxane. That sequence has already been, in a sense, adopted by the Intergroup in SWOGSO221, which compares every two-week doxorubicin/cyclophosphamide (AC) to a weekly low-dose doxorubicin and daily oral cyclophosphamide.
Both regimens are followed by a taxane, either low-dose weekly or standard every two-week paclitaxel. It’s a two-by-two factorial design that doesn’t actually test dose density because variations in dose size, number of cycles and the length of treatment intervals are used. Rather, it is testing metronomic chemotherapy to determine the optimal schedule for paclitaxel.
Another important trial using dose-dense chemotherapy is CALGB-40101, which incorporates the every two-week schedule comparing paclitaxel to AC in patients with high-risk, node-negative breast cancer. It also compares four cycles versus six, and although many clinicians think they already know which is better, this is the first point-on testament.
If you accept my premise that the dose density issue is a continuum, it’s not so difficult to believe that therapy every two weeks is better than every three weeks. One may question whether it’s worth the effort, but because treatment is completed faster and it lowers the risk of neutropenic fever, I believe it’s worth it.
The impact of dose reductions and delays
A retrospective analysis of CMF from Bonadonna in Milan showed that reductions to below 85 percent of planned dose intensity are detrimental to patient outcome, yet interesting evidence from Germany and the United States shows that oncologists lower and delay dose far more often than anticipated (1.6).
Several factors cause dose reduction and delays. To begin, the use of growth factors represents a financial and technical barrier. Also, we didn’t have convincing data that delaying a few days here and there mattered until recently. In addition, toxicities other than myelosuppression, including fatigue, mucositis, diarrhea and nausea, can lead to dose reductions and delays.
Every time we dose-reduce or delay, we may be compromising therapy. Clinicians should ask themselves whether they have evidence that this is safe to do. Right now, they don’t. All the evidence we have says that dose reductions and delays are not safe.
Select publications
 |
Dr Hudis is Chief of Breast Cancer Medicine Service for the Solid Tumor Division of Memorial Sloan- Kettering Cancer Center in New York, New York. |
|
