| You are here: Home: BCU 5|2004: Melody A Cobleigh, MD
Melody A Cobleigh, MD |
EDITED COMMENTS |
 Multigene assay for predicting recurrence in patients with nodenegative breast cancer Multigene assay for predicting recurrence in patients with nodenegative breast cancer
Based on literature review and known prognostic factors in breast cancer, approximately 185 genes were selected for a multigene panel and tested in two data sets: one from Rush-Presbyterian- St Luke’s Medical Center and the other from Providence-St Joseph Medical Center. Twentyone genes appeared to predict for outcome and were then confirmed in a subset of the patients from the NSABP-B-20 tamoxifen-only arm.
NSABP-B-14 tested this multigene panel prospectively in 668 patients with ER-positive, node-negative breast cancer, and the panel predicted recurrence risk far better than age, tumor size or tumor grade. This assay assigns patients a recurrence score from zero to 100 to assist in deciding on treatment alternatives (4.1).
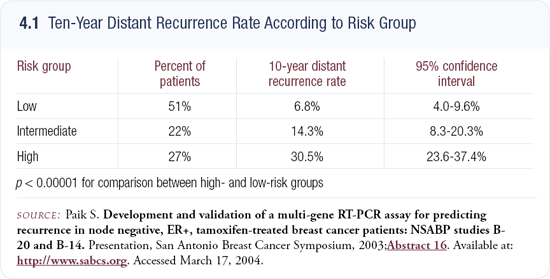
For example, patients at low risk who have approximately a 6.8 percent recurrence risk after five years of adjuvant tamoxifen would realize perhaps a two percent absolute benefit from chemotherapy, whereas patients at high risk would experience a greater reduction. I believe it’s going to make difficult decisions like this much easier for patients and physicians.
Currently, I don’t press patients with node-negative, ER-positive disease to take chemotherapy because I don’t know who really needs it. Rather, I provide the patient with the information and encourage her to discuss it with her family and let me know her decision. In my practice, almost all the young women take chemotherapy, and almost all the elderly women choose not to, but many patients are in the middle.
Clinical impact of dose-dense chemotherapy
I believe the dose-dense approach is an advance in treatment. It’s amazing that chemotherapy every two weeks rather than every three weeks can be less toxic, but that’s been my experience. Prior to this data, my nonprotocol treatment for patients with node-positive disease consisted of AC times four followed by paclitaxel for four cycles.
With dose-dense therapy, dose delays do not occur, the patients feel fine and are thrilled to finish therapy earlier, and neutropenic fever is rare. The one toxicity that concerns me is neurotoxicity because it’s less objective. We can harm patients by continuing paclitaxel when significant neurotoxicity is present.
Proposed NSABP trial of trastuzumab as a radiosensitizer in patients with HER2-positive DCIS
Ductal carcinoma in situ is HER2-positive more frequently than invasive cancers. Theoretically, if we intervene earlier in the pathogenesis of breast cancer, we might be able to prevent HER2-positive breast cancers. Also, in vitro evidence indicates that trastuzumab is a radiosensitizer and a chemosensitizer, so an NSABP study has been proposed in which patients with HER2-positive DCIS will be randomly assigned after lumpectomy to receive radiation with or without concurrent trastuzumab.
While nearly half of ER-negative cases overexpress HER2, only 19 percent of ER-positive cases do so. This proposed trial will evaluate both subsets. Trastuzumab will be administered with one dose at the beginning of radiation and a second dose three weeks later, which should be tolerable based on the ongoing adjuvant trials in which a couple thousand patients have received concurrent trastuzumab and radiotherapy with no safety signals. I’m hopeful we will see fewer ipsilateral recurrences in this trial, and it will be interesting to see whether trastuzumab can prevent HER2-positive DCIS in the contralateral breast.
Clinical trials of adjuvant trastuzumab
Combining the Intergroup and NSABP adjuvant trastuzumab trials is a terrific idea because we’ll have data earlier, hopefully within three years. I’ll be shocked if the trastuzumab arm doesn’t prove to be superior. As for safety, I don’t believe trastuzumab causes a marked increase in cardiac toxicity. The three adjuvant trials currently underway are all monitored every six months, and no significant safety signal has been reported. I don’t use adjuvant trastuzumab in a nonprotocol setting. I believe the oncology community has learned from the bone marrow transplant experience.
An interesting paper presented at the 2003 San Antonio Breast Cancer Symposium evaluated neoadjuvant trastuzumab in primary breast cancer. The data indicated that trastuzumab markedly increases the rate of apoptosis, so it appears to cause cell kill rather than to decrease proliferation. It also pointed out that the apoptosis occurs very quickly, so indefinite long-term therapy may not be necessary.
Combination regimens with bevacizumab
A Phase I/II trial at UCLA is evaluating the combination of trastuzumab and bevacizumab, which is a great idea because HER2-positive tumors significantly activate angiogenesis.
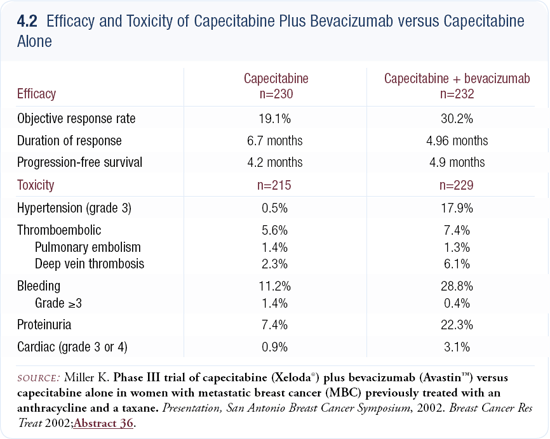
The prior trial of capecitabine with or without bevacizumab is negative from a scientific standpoint because the primary endpoint — time to progression — was not met (4.2). However, the response rate was increased. In the preceding Phase I/II study, the response rate for single-agent bevacizumab was approximately nine percent in heavily treated patients, which is similar to what was seen with trastuzumab in heavily pretreated populations.
ECOG-2100, the current Phase III randomized trial of paclitaxel with or without bevacizumab in patients with locally recurrent or metastatic breast cancer, will be the acid test.
I expect that the trial will be positive, because why would bevacizumab work in colon cancer but not other solid tumors? It’s the same target. In my own small cohort of patients, the patients who were randomly assigned to the combination of paclitaxel/bevacizumab appear to be doing very well compared to the patients receiving paclitaxel alone.
Trastuzumab for locally advanced or inflammatory breast cancer
In patients with locally advanced or inflammatory breast cancer, I believe trastuzumab may be appropriate in the nonprotocol setting. These are patients in big trouble, and no prospective randomized trials are currently evaluating this issue. I believe they need trastuzumab, and I give it to them in combination with chemotherapy. I would probably use the weekly regimen of carboplatin/paclitaxel/ trastuzumab, and if a patient has had local therapy, I’d use radiation concurrent with trastuzumab and then stop.
Nonprotocol management of patients with ER-negative, HER2- positive metastatic disease
Approximately 90 percent of my patients with ER-negative, HER2-positive metastatic breast cancer receive single-agent trastuzumab as first-line therapy. If a patient presents with a life-threatening illness or progresses on trastuzumab, I add chemotherapy. In patients who respond and then progress again, I continue trastuzumab indefinitely and change chemotherapy agents as needed.
The trial that documented a survival advantage for chemotherapy plus trastuzumab, compared to chemotherapy alone, was missing a third arm — trastuzumab alone. A concurrent trial evaluated first-line, single-agent trastuzumab in patients with metastatic breast cancer, and the survival curves of that single-arm trial are identical to the combination arm of the randomized trial.
Even when you evaluate variables such as prior use of adjuvant therapy, patient’s age or number of metastatic sites, the survival curves are identical. Even though the data supporting single-agent trastuzumab are not from the same randomized trial, I believe it exists in a concurrent fashion.
Nonprotocol management of patients with ER-positive, HER2- positive metastatic disease
In patients with ER-positive, HER2-positive metastatic breast cancer, I use frontline hormone therapy, assuming they don’t present with life-threatening disease. If the patient responds and then progresses, I continue with endocrine therapy.
If she does not respond initially, then I use trastuzumab monotherapy and add chemotherapy when progression occurs. I haven’t used trastuzumab and hormonal therapy together because I’m unaware of in vitro models showing a synergy between these two therapies.
When using trastuzumab as monotherapy or in combination with chemotherapy, I use the every three-week schedule. In terms of chemotherapy, when a patient presents in visceral crisis, I find the weekly carboplatin/paclitaxel/trastuzumab combination is extremely well-tolerated and very active. I use either vinorelbine/ trastuzumab or weekly carboplatin/paclitaxel/trastuzumab.
Capecitabine in the metastatic setting
In metastatic disease, I believe sequential single-agent chemotherapy is a gentler approach than combination therapy with equivalent survival. Capecitabine is probably my favorite drug in this setting because it’s oral, very active and extremely well-tolerated as long as patients are properly educated about side effects. I prefer capecitabine before an anthracycline or a taxane in a patient who hasn’t received either one.
As for dosing, I start with full-dose capecitabine — 2,500 mg/m2 rounded down to the nearest 500 milligrams (for 14 days followed by seven days off therapy). I participated in the capecitabine with or without bevacizumab trial in which the FDA mandated starting with the full dose, and I learned that some patients tolerate very large doses of capecitabine. My nurse practitioner is meticulous in educating patients to stop as soon as they begin experiencing side effects. Many patients require a dose reduction, but they need not become extremely ill before we do so.
Select Publications
 |
Dr Cobleigh is a Professor of Medicine and the Director of the Comprehensive Breast Center at Rush University Medical Center in Chicago, Illinois. |
|
