| You are here: Home: BCU 5|2004: Jeffrey Abrams, MD
Jeffrey Abrams, MD |
EDITED COMMENTS |
 Cancer Trials Support Unit Cancer Trials Support Unit
Over the past five years, the Cancer Trials Support Unit (CTSU) (http://www.ctsu.org/) has developed a single regulatory support system. Instead of oncologists who belong to multiple cooperative groups having to register and file different applications every year with each group, they register once and each cooperative group utilizes that information. The centralization of that data has been very helpful. Similarly, centralization of all the IRB data on a per-study basis has been very helpful.
This should ease the burden of clinical trial participation on investigators in the community and academic institutions, which was one of our charges five years ago when the Armitage and the Implementation Committees reviewed and made some important recommendations about the NCI clinical trials system.
Our goal is to increase the speed in which we complete important trials. The system can clearly do that, as witnessed by the recent MA17 trial evaluating letrozole after adjuvant tamoxifen. More than 5,000 patients enrolled in the MA17 trial, and although NCI of Canada led that trial, 3,500 of the patients enrolled were from the United States cooperative groups. We completed accrual to that trial in less than four years and had results about one and a half years later. The system does work, and it can rapidly provide answers to important questions.
We want the different cooperative groups to be competitive in coming up with the best trial ideas — that’s healthy for the system. On the other hand, as soon as those trials are formulated and made available to doctors and patients, it’s very important that they accrue as rapidly as possible. By putting the trials on the CTSU menu, we keep all the advantages of the cooperative groups in terms of their scientific creativity while breaking down the barriers to rapid accrual by allowing cross-group accrual.
Now we’ve basically made every study an Intergroup study, because any member of one adult cooperative group can participate in other cooperative groups’ studies. This becomes important when evaluating the science; as we find molecular signatures and break patients into smaller subsets, more participants and sites will be necessary to obtain the numbers required for these subsets.
Similarly, to do trials in less common diseases, we need this cross-group participation. In its first two years, the CTSU had a slow take-off, but more recently, accrual has been improving.
Barriers to clinical trial accrual
According to the Harris poll presented by Bob Comis at ASCO some years ago, approximately 60 percent of patients claimed to have never been offered participation in a clinical trial. Clearly, if the doctors don’t offer clinical trial participation, we can’t even reach first base. Barriers to clinical trial accrual are multifactorial, and the CTSU was designed to attack several of these barriers (3.1).

Having the infrastructure support — the research nurse support, the IRB support and the financial support — to actually carry out the research is critical when deciding to participate in clinical trials — especially in community practice. In addition, sometimes randomization can be a problem for some physicians and patients. While not able to handle all those issues, the CTSU was designed to reduce the burden of the regulatory paperwork.
The CTSU is dependent upon Congress for its budget, and we are trying to show data that clinical trial research costs more, on average, than the $2,000 per patient that we typically reimburse for Phase III trials. Hopefully we’ll be able to make that case and obtain better reimbursement. One of the other strategies we use is to work closely with industry partners on several important studies. Sometimes they help us supplement the research reimbursement, and some of our trials are reimbursed at higher rates than the government reimbursement rate.
Physicians’ perspectives on randomized trials
For many physicians who choose not to participate in clinical trials, randomization is an issue. We, as physicians, feel that we know the right answer, although time and again the trials have shown that we don’t know the right answer or that our initial intuition isn’t correct. Many physicians like to go with their bias or intuition and don’t want to randomly assign patients to therapy. In addition, randomization takes more time on the part of the physician. They must explain the pros and cons, as opposed to just presenting a patient with a definitive treatment plan.
It takes a special type of physician who’s willing to put biases aside and take the necessary time to explain why the choice of the therapy will be assigned randomly and why that makes sense in this situation. We always have a harder time when the trial is comparing a treatment to no treatment. The physicians who utilize a particular treatment are biased that the treatment will work. Those trials are quite challenging, especially in radiation oncology.
Clinical research in elderly patients with breast cancer
Elderly patients in this country — including patients with breast cancer — are difficult to enroll into clinical trials. All the barriers in younger people plus the additional barriers of travel, supportive care at home and, perhaps, different approaches to the idea of randomization exist amongst the elderly. We must design the appropriate trials and then educate the doctors and the patients about the need to have these patients participate. In the United States, our population is aging. In coming years, the elderly are going to be the largest number of patients with cancer, and we need evidence-based medicine to treat them properly. If we don’t do clinical trials in that group, we won’t have that.
CALGB-49907: Adjuvant chemotherapy trial in the elderly
CALGB-49907 is not currently accruing well. Hyman Muss has made some changes to try to make the eligibility a little more streamlined and easier for physicians and patients. Unfortunately, we ran into toxicity problems in two patients in the capecitabine arm. These cases were evaluated by the data monitoring committee, and one case was thought to be related to an enzyme deficiency. The other case was thought to be an unfortunate late toxicity in which the patient didn’t contact the physician in a timely fashion.
New rules have been written into the trial to ensure toxicity problems do not occur again. We strongly believe that this trial will address a very good question: How does an oral agent compare to traditional intravenous chemotherapy? In patients with metastatic disease, capecitabine has been shown to be better than CMF, so we might even have an efficacy advantage.
IMPACT trial: Neoadjuvant hormonal therapy
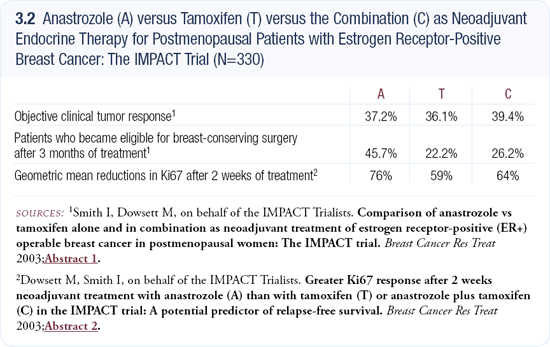
Neoadjuvant chemotherapy and neoadjuvant hormonal therapy offer great potential advantages. If we can find surrogate markers to predict outcome, we can speed up, by many years, the ability to determine which treatments work in the adjuvant setting. The investigators from the IMPACT trial (3.2) — comparing anastrozole, tamoxifen, and anastrozole plus tamoxifen — were trying to make that point. In terms of reducing Ki67, anastrozole was better than tamoxifen, which parallels the ultimate outcome of the ATAC trial.
I don’t believe in using a single marker as the only surrogate. However, if we can use a surrogate marker to predict the ultimate outcome and correlate it with survival, then these trials may not need to enroll 3,000 to 5,000 patients. Instead, they can enroll 300 to 400 patients and provide an answer within a year. Now we need to prove that surrogates correlate with survival, and the IMPACT trial was an interesting first step in that direction.
The IMPACT trial seemed to confirm that the aromatase inhibitors might be better than tamoxifen in patients with HER2-positive disease. It could be that the benefit associated with anastrozole in the ATAC trial was largely due to the population with HER2-positive disease, and tamoxifen and anastrozole may be equally effective in patients who don’t overexpress HER2. It’s also possible that anastrozole is better even in the patients with HER2-negative disease. I would like to see that analysis of the ATAC trial data.
Impact of CALGB-9741 on ongoing adjuvant trials
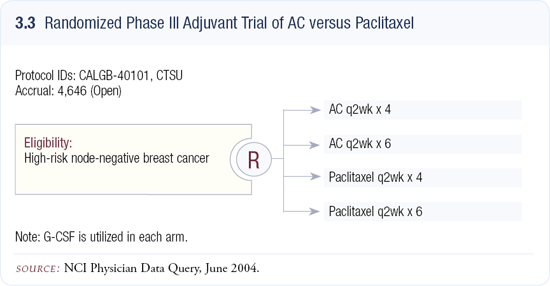
After the presentation of the results from the adjuvant dose-dense trial (CALGB- 9741), the cooperative groups had to decide whether they should modify any of the ongoing adjuvant trials using a doxorubicin, cyclophosphamide and taxane combination. With regards to the adjuvant trastuzumab trials, they decided not to modify them because it was not known what the interaction would be between trastuzumab and this altered doxorubicin schedule. However, in the CALGBled Intergroup trial (CALGB-40101) — comparing AC for four or six cycles to paclitaxel for four or six cycles — in women with node-negative disease, the AC schedule was changed to every two weeks (3.3).
Although we believe CALGB-9741 is a positive study, it is a single positive study. Some do not believe adjuvant dose-dense therapy should become the new standard based on one study, especially when fairly small differences in survival were reported. Therefore, they want another study to test this concept before it becomes a standard approach. I agree, because we have sometimes seen small differences in survival not hold up over time. Personally, I believe the results from CALGB-9741 will hold up, but it’s certainly reasonable to wait for a confirmatory study. The NSABP will try to address this again by comparing dose-dense AC followed by T to ATC in a head-to-head comparison.
Adjuvant bisphosphonates
The largest study conducted to date on this issue was done in Europe and showed a survival benefit for adjuvant clodronate. However, adjuvant bisphosphonates didn’t really catch on and become the standard of care because the benefit in reduction of bone metastases did not hold up. We are awaiting the results from NSABP-B-34 before concluding whether adjuvant bisphosphonates have a role as standard therapy. In follow-up to NSABP-B-34, a SWOG-led trial will compare more potent bisphosphonates to clodronate. The bisphosphonates and the aromatase inhibitors make sense as combination therapy because the bisphosphonates prevent osteoporosis. Ongoing trials — both NCI- and pharmaceutical company-sponsored — will determine the efficacy of the bisphosphonates in preventing osteoporosis related to the aromatase inhibitors.
Select publications
 |
Dr Abrams is the Acting Chief of the Clinical Investigations Branch of the National Cancer Institute’s Cancer Therapy Evaluation Program in Rockville, Maryland. |
|
