| You
are here: Home: BCU 5|2003: Mark
D Pegram, MD
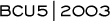
Edited comments by Dr Pegram
Early Phase I experience with trastuzumab/cisplatin
in metastatic disease
One important case we have followed is a patient in her mid-50s,
who was diagnosed with metastatic breast cancer about 10 years
ago. She had multiple pulmonary nodules and a supraclavicular lymph
node, which was biopsied and confirmed that she had distant metastases.
At the time, HER2 testing was in its infancy, and there were no
commercially available tests. An IHC assay was run in Dennis Slamon’s
laboratory, and the tumor was scored as IHC 3+.
Consequently, the patient was offered participation in one of
the early Phase I trastuzumab clinical trials, evaluating a combination
of chemotherapy plus trastuzumab. The combination she received
was trastuzumab/cisplatin. After receiving a few cycles of cisplatin
and maybe 18 or 20 weeks of trastuzumab, she had a complete clinical
remission. Back in those days, Phase I trials came to an end, and
she went off study.
That was more than 10 years ago. She is alive and well and in
complete remission to this day. We all have miraculous patients
like this who do particularly well. Is this just an interesting
anecdote or is this patient trying to tell us something about the
biology of breast cancer? In particular, the interaction between
the platinums and trastuzumab is something we have been studying
in our laboratory at UCLA for quite some time. We would like to
think that the synergy between these two agents explains this patient’s
particularly good outcome.
Phase III randomized trial of trastuzumab/paclitaxel
with or without carboplatin
The in vitro synergy between the platinums and trastuzumab has
recently been put to the test. At the San Antonio Breast Cancer
Symposium, Dr Nicholas Robert presented the results from a study
that randomized patients with HER2- positive metastatic disease
to receive trastuzumab/paclitaxel or trastuzumab/ paclitaxel/carboplatin.
The results were remarkable. In the patients who received carboplatin
in addition to trastuzumab/paclitaxel, the response rates and the
time to progression were significantly improved.
Since FISH status was analyzed retrospectively and not all of
the patients have had their tumors tested by FISH, preliminary
data revealed a trend in the FISHpositive patients towards prolonged
survival with the addition of carboplatin to trastuzumab/paclitaxel.
This is very provocative data suggesting that addition of carboplatin
might improve the survival of patients with HER2-positive metastatic
breast cancer.
Trastuzumab/platinum/taxane regimens
At UCLA, we have an ongoing confirmatory trial comparing trastuzumab/
docetaxel to trastuzumab/docetaxel/carboplatin. The trial is based
on data we generated at UCLA showing that the synergy between trastuzumab
and docetaxel appears to be better than the synergy between trastuzumab
and paclitaxel. Therefore, patients at UCLA are encouraged to consider
enrolling in that clinical trial.

If, however, patients decline participation or don’t meet
the strict eligibility criteria but might still benefit, we have
in some instances treated them off protocol with a triple-drug
regimen. We have had encouraging results even off protocol, and
our colleagues in the community are also seeing good success off
protocol.
There is also a neoadjuvant trial evaluating a trastuzumab/platinum/taxane
regimen. At the last couple of ASCO meetings, Judith Hurley has
reported preliminary data showing particularly high pathologic
complete response rates in patients treated with neoadjuvant trastuzumab/docetaxel/cisplatin.
Even off protocol, I think this regimen is a consideration. As
oncologists, we are very comfortable using taxane/platinum combinations.
We’re very comfortable with the types of side effects encountered,
particularly cytopenias. We saw significant cytopenias with these
trastuzumab/platinum/taxane regimens, more so than with just trastuzumab/taxane,
but they were certainly manageable. In Robert’s study, the
incidence of febrile neutropenia was four percent in one of the
arms and five percent in the other arm, but there were no statistically
significant differences.
First-line therapy for patients with HER2-positive
metastatic disease
Published data in the New England Journal of Medicine show that
trastuzumabbased chemotherapy combinations prolong survival. How
many drugs have been shown to improve survival in patients with
metastatic breast cancer? Anthracyclines, for example, have not.
The meta-analysis of the anthracycline studies in metastatic disease
failed to document a survival advantage with any statistical confidence.
It is really hard to dismiss that data and not use trastuzumab
as first-line therapy for patients with metastatic disease.
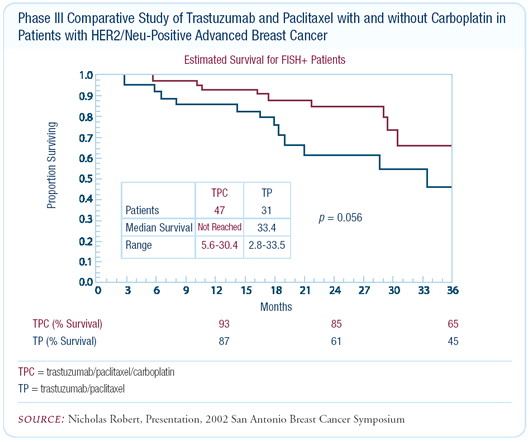
BCIRG-006 adjuvant trastuzumab trial
BCIRG-006 is a multinational, randomized, controlled trial for
patients with FISH-positive, early stage breast cancer — either
node-positive or high-risk, node-negative disease. Patients are
randomized to one of three different treatment arms: AC followed
by docetaxel, AC followed by docetaxel/ trastuzumab with trastuzumab
continued for a total of one year, and trastuzumab/docetaxel with
either carboplatin or cisplatin.
For the first time in a large randomized adjuvant study, a nonanthracyclinecontaining
synergistic combination will be put to the test in a very carefully
selected patient population. All of the patients must have FISH-positive
disease, therefore, I think the trial will define the standard
of care for the adjuvant treatment of patients with HER2-positive
breast cancer.
BCIRG-006 is accruing well ahead of schedule. In fact, more than
1,500 of the anticipated 3,150 patients have been accrued to the
trial so far. It will likely be the first adjuvant trastuzumab
trial to complete accrual. It is anticipated that the accrual will
be closed at the end of 2003 or early first quarter 2004.
The other important component of this trial is safety. There
is a data safety monitoring committee and a specific cardiac safety
monitoring committee. They are monitoring all of the treatment
arms in real time, and they have predefined trigger points that
call for an interruption in the protocol if there are any flags
for cardiotoxicity in the AC followed by trastuzumab/docetaxel
arm.
In fact, the study was designed in such a way that the arm can
drop out. If we encounter cardiotoxicity problems, we would still
have a two-arm study — one arm with conventional chemotherapy
and the other arm with trastuzumab/ platinum/taxane.
It doesn’t appear that cardiac safety is going to be a big
issue in the adjuvant trastuzumab trials. Although there was a
scare some months ago with the Intergroup trial and one arm was
closed temporarily, that arm has reopened and the most recent update,
presented by Dr Edith Perez, reveals that the incidence of depressed
ejection fractions is the same in all of the arms of the Intergroup
trial.
Next page
Page 1 of 2
|
