You
are here: Home: BCU 5|2003: Paul
E Goss, MD, PhD, FRCP(CA),
FRCP(UK)
Edited comments by Dr Goss
Development of resistance to endocrine therapy
There are two types of resistance to endocrine therapy in invasive
breast cancer — de novo resistance and acquired resistance.
We’re only beginning to understand de novo resistance. Kent
Osborne and his colleagues presented their ongoing research on
HER2 overexpression, showing that through HER2, the mitogen activated
protein (MAP) kinase pathways increase, and through MAP kinase,
a number of receptors are phosphorylated.
Growth stimulation occurs through MAP kinase independently, but,
in addition, MAP kinase seems to alter the estrogen receptor, making
it more sensitive to both circulating estrogen and to the agonistic
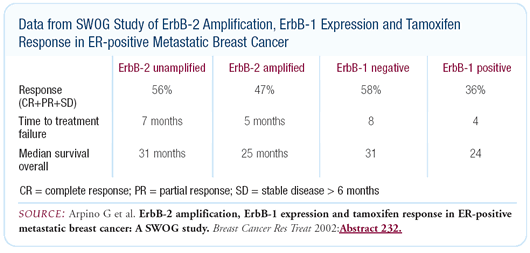
effects of tamoxifen. In a neoadjuvant study, Matt Ellis and his
colleagues showed that patients who have HER2-positive, estrogen
receptor-positive tumors are de novo resistant to tamoxifen but
are sensitive to aromatase inhibitors. It’s probably partly
through the MAP kinase pathway.
Development of aromatase overexpression
Endocrine resistance may occur in a number of ways. One is through
estrogen receptor mutation at the breast cancer cell level and
another is through the sensitivity of the cell to the estrogen
content in and around the tumor cell. If one thinks about estrogen
arriving at the breast cancer cell, there’s collaboration
between the epithelial cell and the peritumoral stromal cells.
Aromatase is functional in the peritumoral and the tumor cells,
and it recruits the help of adjacent cells to create an autocrine
loop of estrogen production. That loop has been shown to happen
by estrogen deprivation and specifically through aromatase inhibitor
therapies.
In cell culture you can evoke aromatase overexpression by long-term
estrogen deprivation (LTED). The LTED cells will try to overcome
the inhibition and upregulate aromatase. The same thing is true
if you use aromatase inhibition in vivo in animal models. Tumor
cells can also make substances that create alternative promoters
of the aromatase gene. The aromatase gene is supposed to use specific
promoters in the breast, and under influence of these substances,
the ovarian promoter, for example, can be de-silenced, and the
gene starts to function as it does in the ovary and drive aromatase
production.
A phenomenon of aromatase overexpression in the face of aromatase
inhibition and estrogen deprivation potentially occurs in the tumor
and peritumoral cells. Couple that with a MAP kinase pathway being
overexpressed, and you have increased sensitivity of the receptor
resulting in exquisite sensitivity to estrogen and to the agonistic
effects of any of the SERMs.
Optimal duration of adjuvant aromatase therapy
We’ve learned that prolonged therapy with tamoxifen may
lead to a form of tamoxifen-acquired resistance, but we don’t
yet know if this occurs with aromatase inhibitors. We also don’t
know the correct duration of therapy with aromatase inhibitors.
One clue might be the number of patients who relapse during therapy;
another might be seeing what happens to patients at the cessation
of adjuvant aromatase inhibitor therapy. That’s an unknown
entity at this point, and we don’t yet have data from ATAC
or any other aromatase trial to tell us what’s going to happen.
For years, investigators have talked about designing trials in
which one switches back and forth between anti- and pro-estrogenic
therapies to see if one could confound the cell. It has been shown
that physiologic levels of estrogen can destroy tamoxifen-sensitive
cells. Theoretically, the same thing could happen with aromatase
inhibitors. If you supersensitize cells to estrogen and then increase
the concentration of estrogen, it might be cytocidal, or cytostatic.
Therefore, re-introducing estrogen therapy after aromatase inhibitors
might work — and it might be effective at lower doses than
previously used.
Utilization of agents to reverse resistance to
aromatase inhibitors
It’s possible that prolonged aromatase inhibitor therapy
alone will control most tumors, or we might find a point at which
we need to introduce a resistance reverser. With de novo resistance,
we might need to couple the inhibitor with a reverser from the
beginning. Gefitinib (Iressa®) is an obvious reverser — it
blocks the EGF receptor tyrosine kinase and it appears to reverse
the acquired estrogen deprivation resistance that occurs — so
an obvious combination would be an aromatase inhibitor with gefitinib.
Whether they would be given in sequence or together from the beginning
will have to be studied in clinical trials.
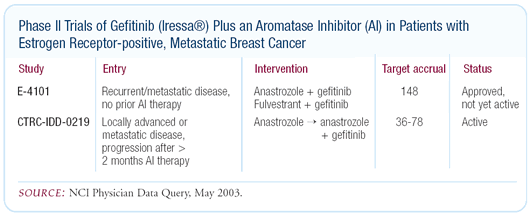
Currently there are no adjuvant trials evaluating that combination,
but there are metastatic trials with gefitinib plus an aromatase
inhibitor. There are also trials being designed in which patients
failing on an aromatase inhibitor are either switched to gefitinib
or continued on the aromatase inhibitor plus gefitinib.
Next page
Page 1 of 2
|
