| You
are here: Home: BCU 5|2003: Mark
D Pegram, MD
Adjuvant trastuzumab in the nonprotocol setting
In the nonprotocol adjuvant setting, it’s hard to know
the right thing to do. I’ve evaluated patients with high-risk
disease — 10 or more positive nodes — in whom I’ve
considered adjuvant trastuzumab therapy off protocol.
I don’t want to say that this is something that is widely
done at our center — it’s infrequent and uncommon.
However, the prospects for a patient with that type of disease
are really unacceptable. If you consider that trastuzumab prolongs
survival in patients with metastatic disease, biologically there
are probably many similarities between high-risk Stage II and advanced
disease. Therefore, that would be an interesting patient population
to study, and off protocol we have considered such patients for
adjuvant trastuzumab therapy.
Influence of trastuzumab therapy on tumor HER2
status
We don’t really know what happens to a patient’s
HER2 status after they have been treated with trastuzumab. In the
metastatic setting, some case series of preand post-treatment biopsies
have been reported with conflicting results. Because most of the
trastuzumab trials have been conducted in patients with metastatic
disease, in whom it is difficult to obtain biopsies, there is no
good database of pre- and post-treatment tumor tissues.
When HER2 gene amplification occurs, it appears to be a very
stable event. Several studies have shown good concordance between
the HER2 status in the primary tumor and the metastases. Given
that level of concordance and the presumed genetic stability for
HER2 amplification, I would be very surprised if trastuzumab could
change HER2 gene amplification.
I suspect that if one rebiopsied a patient with residual tumor
after trastuzumab therapy, one would find the HER2 gene still amplified.
I would expect that the tumor’s genotype would probably not
be changed whether the cancer was responding or resistant to trastuzumab.
It’s just mind-boggling that we haven’t done that yet.
We need to do a better job of obtaining tissue for laboratory analysis.
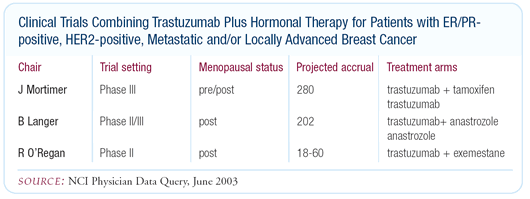
Trials combining trastuzumab with hormonal therapy
In preclinical models, we observed greater efficacy for tamoxifen
plus trastuzumab and fulvestrant plus trastuzumab compared to each
drug alone. The more we can do to constrain potential mechanisms
of escape for the cancer cell, the better. If HER2 is a potential
mechanism of escape from hormone sensitivity, then targeting both
at the same time could work.
A number of ongoing trials are evaluating these combinations,
and based on our preclinical data at UCLA, we think they might
work. However, due to the inverse correlation between HER2 and
ER, accrual to these studies has been difficult.
Fulvestrant in clinical practice
I’ve been pleased with fulvestrant and have not found the
need to deviate from the package insert recommendations. In my
experience, patient tolerance has been excellent with very few
complaints about side effects. We’re using fulvestrant in
patients who have already had prior hormonal therapies, so perhaps
they don’t mention side effects because they are already
used to the hormone withdrawal side effects.
I’ve certainly not had the occasion to stop fulvestrant
in any patient because of toxicity. Compliance is very good, and
the injection really isn’t an issue. These are highly motivated
patients with a devastating disease, so they do not object to receiving
an injection. I am using two 2.5 cc injections.
Potential synergy between fulvestrant and trastuzumab
HER2 does two different things to the estrogen receptor (ER).
First, it decreases ER expression so there’s less ER in a
patient with HER2-positive disease. Even if the tumor is ER-positive,
it is less positive than a tumor from a patient with HER2-negative
disease. Second, through cross talk between the signal transduction
pathways for HER2 and ER, there is phosphorylation of the ER that
may alter the biology of the receptor and result in an activated
species. For that reason, it would be nice to eliminate the ER
in a HER2-driven tumor. Fulvestrant, given its mechanism of action,
provides a particularly attractive way to deal with the ER in a
patient with HER2-positive disease. It’s an ideal model that
works in preclinical xenograph models.
Trials combining trastuzumab with biologic agents
Erlotinib
We are especially interested in moving forward with biologic
combinations. In fact, we have a couple of open trials at UCLA
evaluating combinations of biologics. Dr Carolyn Britten is conducting
a Phase I/II clinical trial with trastuzumab in combination with
erlotinib, a small molecule inhibitor of the epidermal growth factor
receptor (EGFR/HER1).
In this instance, we’re studying whether inhibiting HER1
and HER2 simultaneously might be better than just HER2 blockade
alone. This may potentially allow fewer avenues of escape for the
cancer cells. We hope this will be another improvement in the treatment
of HER2, positive disease.
Bevacizumab
Based on measurements from our laboratory showing a strong correlation
between HER2 and expression of the vascular endothelial growth
factor (VEGF), a Phase I trial just opened at UCLA. A very strong
concordance between HER2 and VEGF in primary breast cancer has
been confirmed by other groups. Linderholm et al presented data
at ASCO demonstrating the same thing in a very large data set.
We’ve just completed a study involving about 612 patients
with primary breast cancers showing this type of correlation.
Part of the pathophysiology behind HER2-driven disease may be
regulation of the angiogenic switch. If we could address both of
those problems, maybe we would see improved therapeutic efficacy.
We’ve applied this theory in animal models using a combination
of trastuzumab and the humanized anti-VEGF antibody, bevacizumab.
In those studies, the combination had better results against murine
tumor xenographs.
Based on these pilot data, we have a new Phase I trial that will
be escalating the bevacizumab dose and using the standard FDA-approved
dose of trastuzumab. When we complete the Phase I trial, it is
designed to roll over into a formal Phase II trial to accrue more
safety and efficacy data.
Treatment options for patients with ER-negative,
HER2- negative, metastatic disease
This is a difficult subject, because it involves the controversy
over combination chemotherapy and monotherapy. For the first time,
the FDA has approved a combination chemotherapy regimen for metastatic
disease, the docetaxel/capecitabine combination.
In appropriate cases, I think that combinations like this can’t
be overlooked. In my practice, I’ve moved towards combination
chemotherapy for patients with potentially life-threatening metastatic
disease; otherwise, the off-protocol treatment for patients with
ER-negative, HER2-negative disease involves sequential single-agent
regimens.
Based on cross-trial comparisons of Phase II data, many single
agents have very similar response rates and times to progression.
Given the relative equivalence of capecitabine, vinorelbine and
gemcitabine in patients who have failed a taxane and an anthracycline,
I make decisions based on convenience and toxicity.
If patients have not been treated with an anthracycline or a taxane,
I start with those first. But many of the patients that we’re
seeing now with metastatic disease have already failed an anthracycline
or a taxane in the adjuvant setting, and we have to consider moving
on to different classes, especially if they’ve relapsed quickly.
If they’ve had a long disease-free interval, then we treat
them with either a taxane or anthracycline. However, in the taxane
and anthracycline failures, capecitabine really is a strong consideration
because of its convenience for the patient.
Transition from hormonal therapy to chemotherapy
The transition from hormonal therapy to chemotherapy in metastatic
disease maybe a little bit easier with capecitabine than with other
agents. In patients with low-volume disease and no life-threatening
metastases, capecitabine is an attractive option. We have used
it in elderly patients in whom pulling out the “ big gun” intravenous
drugs is a bit more problematic.
On the other hand, in patients who have marked progression, rising
liver function tests and symptomatic pulmonary metastases, combination
chemotherapy — like capecitabine/docetaxel — becomes
more of a consideration.
Phase III trial comparing capecitabine with
or without bevacizumab
It’s interesting how the trial was viewed. It was touted
as being a negative study, which it was, since it failed to meet
its primary endpoint. However, when looking at a dataset, it’s
very important to see if there are any positive signals.
The response rate for the capecitabine/bevacizumab arm was significantly
higher than for the capecitabine-alone arm. There must be some
explanation for that, because there was a blinded response evaluation
committee. One potential explanation is that the bevacizumab had
a beneficial effect.
We might have missed a significant improvement in time to progression
by virtue of the fact that these were heavily pretreated patients.
Historically, there aren’t many drugs that have been shown
to improve time to progression after anthracycline and taxane failure.
This is a really difficult patient population.
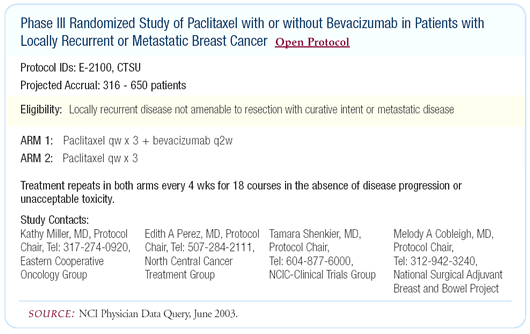
ECOG-E-2100: Phase III trial comparing paclitaxel
with or without bevacizumab
ECOG is conducting a large randomized trial comparing paclitaxel
with or without bevacizumab as first-line therapy for patients
with metastatic disease. I believe that there’s still hope
for bevacizumab in metastatic breast cancer. Until I see the frontline
trial, I’m not going to walk away from the concept of targeting
VEGF with bevacizumab.
Our data demonstrates a correlation between HER2 and VEGF. The
patients with the highest probability of responding to VEGF-directed
therapy are the HER2-positive patients. A limitation of the ECOG
trial is that patients with very high levels of VEGF might not
be accrued to that trial.
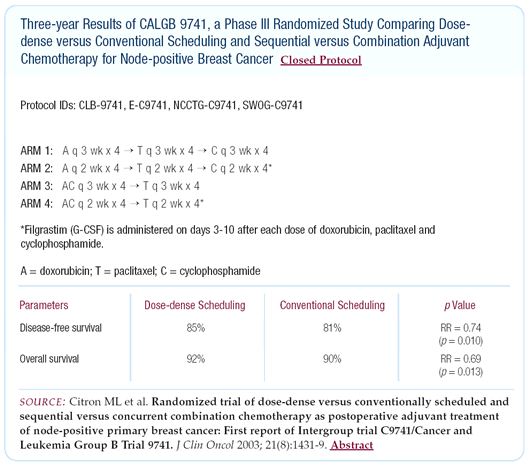
Use of adjuvant dose-dense chemotherapy schedules
The new CALGB-9741 dataset are very provocative. The results
must be explained, and they cannot be dismissed. Clearly, there
was a statistically significant benefit for dose-dense chemotherapy
compared to an every-threeweek schedule. Based on this interim
analysis, the cooperative groups have decided to move to a dose-dense
approach in their future and, in some cases, ongoing studies.
I would like to see confirmation in other clinical trials, and
confirmatory trials are in progress, so we will have that data
in the future. Off protocol, should we be taking this approach
into consideration for the treatment of our patients? It is an
attractive option for a patient with a high risk of recurrence
(i.e., Stage II disease).
I have used the dose-dense approach in some patients, and I have
tweaked the regimens a bit in some cases. For example, I have given
four cycles of the anthracycline-containing combination with growth
factor support and then used weekly taxanes without growth factor
support in patients in whom growth factor use was an issue or in
an elderly patient whom I didn’t want to have as much risk
of neutropenia. Weekly taxanes are still a dose-dense regimen.
In some instances, I’ve used the every-two-week schedule
all the way through.
Select publications
|
