| You
are here: Home: BCU 4|2003: Edith
Perez, MD

Edited comments by Dr Perez
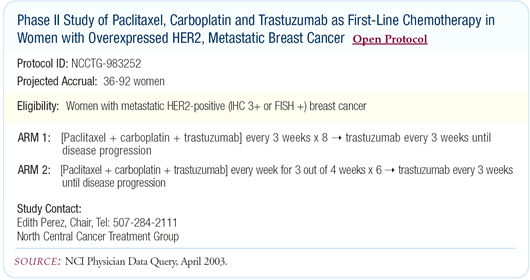
NCCTG-983252: Randomized Phase II trial comparing
two schedules of paclitaxel, carboplatin and trastuzumab
We compared a weekly schedule to a once-every-three-week schedule
of paclitaxel, carboplatin and trastuzumab in patients with HER2-positive
metastatic breast cancer. Tolerability was much better for the
weekly schedule. Although I thought this would be the case, I was
surprised how great the tolerability was for the weekly regimen.
Essentially, there was no significant toxicity and the activity
was very high.
Our trial fits in very well following Nick Robert’s data
demonstrating the benefits of adding carboplatin to paclitaxel
and trastuzumab, administered once every three weeks.
We will present our results at ASCO 2003. The target accrual
for our study was 92 patients, and we will report data on approximately
75 percent of these patients. Because we found the weekly schedule
to be better tolerated, after a certain number of patients enrolled,
we actually closed the once-every-threeweek arm and continued accrual
only to the weekly regimen.
For the weekly schedule, we administered paclitaxel three out
of four weeks. I believe it is critically important to take that
fourth week off of chemotherapy to really optimize tolerability.
In both arms, we administered the chemotherapy concurrently with
trastuzumab for the first six months. Then at the six-month point,
we discontinued the chemotherapy and continued trastuzumab alone — trying
to maximize the activity of the interaction of the three drugs
while ameliorating long-term toxicities.
NCCTG-N9932: Phase II docetaxel and carboplatin
trial
We submitted to ASCO 2003 the results from our Phase II trial
evaluating docetaxel and carboplatin, administered every three
weeks, as first-line therapy in patients with metastatic breast
cancer. There were various reasons for conducting this trial. First
was the activity of docetaxel. Second was the desire to test the
other taxane, and we were the first cooperative group in the United
States to test paclitaxel and carboplatin. Third were data from
the UCLA group and the BCIRG evaluating docetaxel, carboplatin
and trastuzumab, but there was no solid data for the chemotherapy
alone.
Treatment was continued until progression or toxicity. The study
demonstrated that the activity was very comparable to the activity
for paclitaxel and carboplatin. Slightly more myelosuppression
occurred because we did not use prophylactic growth factor support — although
it was allowed. There was very little peripheral neuropathy.

Clinical trials of epidermal growth factor receptor
(EGFR) inhibitors
We are developing new trials to address the issue of anti-EGFR
therapy in patients with metastatic disease. There has been some
preclinical, initial Phase I and Phase II data demonstrating that
there is indeed activity for these drugs. We are going to conduct
a trial evaluating gemcitabine in combination with OSI-774, also
known as erlotinib, in patients with refractory breast cancer.
Within the NCCTG and the rest of the breast Intergroup, we are
also developing a large first-line trial, which will take a few
months to be activated, exploring gefitinib (Iressa®). We plan
to manage patients initially with chemotherapy consisting of docetaxel
and capecitabine. Patients who have at least disease stabilization
will be randomized to receive gefitinib or placebo. We are exploring
the potential for this targeted therapy to maintain the response
seen with initial chemotherapy.
Recently, we’ve seen some exciting results in terms of
survival with the docetaxel/ capecitabine combination, and we are
actually planning to utilize a slightly modified schedule from
the one published by Dr O’Shaughnessy. Although this regimen
is very appealing, the issue of toxicity has prevented many physicians
from incorporating it into their practices. Since that initial
study, other analyses have documented that we can start with lower
doses of the chemotherapy drugs. That is why we want to incorporate
the combination in this new clinical trial.

Sequential single-agent versus combination chemotherapy
in metastatic disease
I’m really happy Dr Sledge published the data from ECOG-1193,
because I believe it will dispel a number of myths. For example,
there is a myth that combination chemotherapy is more toxic and
leads to a worse quality of life than single-agent chemotherapy.
In patients eligible to receive first-line chemotherapy for metastatic
breast cancer, ECOG-1193 demonstrated that the combination of paclitaxel
concurrent with doxorubicin led to a better response rate and time
to progression with a similar quality of life and survival compared
to a sequential taxane and anthracycline regimen. This study supports
the use of combination therapy, because those patients had a higher
possibility of responding and living longer without disease progression
and without an adverse effect on quality of life.
In my practice, if a patient has a good performance status and
symptoms from the malignancy, it makes sense to ameliorate the
symptoms from the tumor as soon as possible, while really paying
attention to tolerability. That’s where there is a big difference
between using good combination chemotherapy and highdose chemotherapy
with transplant, because the latter approach led to high response
rates with significant toxicity.
I believe single-agent chemotherapy is also a very good option
for the patient who is relatively asymptomatic and doesn’t
have rapid disease progression or visceral crisis. It’s not
that I use combination chemotherapy for all patients or that I
insist on single-agent sequential therapy; however, we are planning
to do a study to address this in patients with refractory disease.
Combining capecitabine and irinotecan in patients
with metastatic breast cancer
We conducted a large, multi-institutional, community-based, randomized
Phase II trial that clearly demonstrated the activity of irinotecan.
In a subset of patients with prior exposure to both anthracyclines
and taxanes, the response rate for weekly irinotecan was 27 percent.
We plan to build on these data and the experiences with capecitabine
in the advanced breast cancer setting. We don’t yet know
anything about the combination of capecitabine and irinotecan in
patients with breast cancer, but that’s one of the arms we
will use in our Phase III trial. We will randomize patients with
disease that is refractory to anthracyclines and taxanes to combination
or sequential therapy with capecitabine and irinotecan. We will
focus on time to progression as the main endpoint, while evaluating
quality of life.
I feel that the gastrointestinal toxicity may be somewhat lower
with irinotecan in breast cancer patients compared to colorectal
cancer patients. There are several reasons for that: (1) patients
with colorectal cancer — at least most of the time — have
had surgery on the gastrointestinal tract and that may have an
impact on irinotecan’s tolerability, and (2) in the colorectal
trials, irinotecan is typically combined with 5-fluorouracil, which
can also enhance toxicity. We are taking a different approach in
the breast cancer trials by evaluating irinotecan alone or in combination
with capecitabine.
Fulvestrant in the metastatic setting
We have had an interest in fulvestrant at the Mayo Clinic and
in the NCCTG for many years. We participated in one of the pivotal
trials conducted in the United States, which was eventually published
by Dr Osborne in the Journal of Clinical Oncology.
Fulvestrant is a very well-tolerated drug. It provides an alternative
to oral therapy, which could be very important for patients who
have difficulty remembering to take tablets on a daily basis or
patients who do not have prescription coverage for oral medications.
In patients with estrogen receptor-positive metastatic breast
cancer who have had prior exposure to tamoxifen and aromatase inhibitors,
we have been conducting a Phase II trial, through the NCCTG, evaluating
the activity and tolerability of fulvestrant. Our accrual is going
very well. Activity has been clearly demonstrated, and we have
not had any problems with hot flashes.
Data was presented at the San Antonio Breast Cancer Symposium
by another group demonstrating the feasibility and activity of
fulvestrant after aromatase inhibitors. Hopefully, this larger
clinical trial will corroborate the activity of fulvestrant in
this patient population.

Next page
Page 1 of 2
|
