| You
are here: Home: BCU 4|2003: Edith
Perez, MD

First-line therapy for patients with HER2-positive
metastatic breast cancer
We now have two well-conducted, Phase III randomized clinical
trials comparing the efficacy of a taxane in combination with trastuzumab
to a taxane alone. The combination demonstrates an improvement
in response rate, time to progression and survival.
In patients with HER2-positive metastatic breast cancer, my first-line
recommendation would be a taxane and trastuzumab. Based on the
Robert data, I may add carboplatin. I would not use doxorubicin-based
chemotherapy as first-line therapy.
Continuing trastuzumab after disease progression
In my standard practice for HER2-positive metastatic disease,
I use trastuzumab until disease progression or toxicity. The question
of whether trastuzumab should be continued after disease progression
is one we are wrestling with on a day-to-day basis. No one knows
the answer.
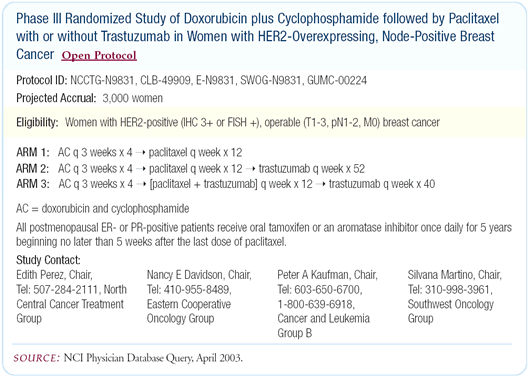
NCCTG-N9831 adjuvant trastuzumab trial
N9831 is a randomized Phase III clinical trial building on several
issues: (1) the relative importance of anthracyclines in the adjuvant
management of patients with HER2-positive breast cancer, (2) the
value of taxanes in patients eligible to receive adjuvant therapy,
(3) the specific value of taxanes for patients with HER2-positive
breast cancer, and (4) the value of weekly paclitaxel therapy for
patients with breast cancer.
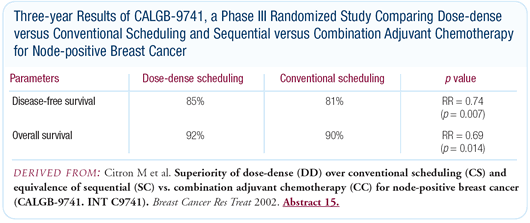
We were comforted by the data presented from CALGB-9741. That
trial administered dose-dense chemotherapy with growth factor support
once every two weeks, and in our trial we are using an even more
dose-dense approach by administering paclitaxel on a weekly basis.
The AC in our trial is still being given once every three weeks.
Although we thought about potentially changing it to once every
two weeks, we are not going to for several reasons.
First, we hypothesized that the advantage seen in CALGB-9741
may be due to the paclitaxel schedule. This theory is partially
based on Marjorie Green’s data at MD Anderson, which evaluated
the benefit of giving weekly paclitaxel compared to once every
three weeks in the neoadjuvant setting. Additionally, we have data
regarding the cardiac safety of AC administered once every three
weeks followed by paclitaxel with or without trastuzumab. We didn’t
want to introduce another factor that could impact on cardiac toxicity.
Right now we feel very comfortable with the schedule. We know
patients in both the control or investigational arms are receiving
dose-dense paclitaxel, which we think is perhaps the most important
aspect of dose-dense treatment.
Cardiotoxicity in the NCCTG-N9831 adjuvant trastuzumab
trial
In January 2002, we received notification of a few patients who
developed congestive heart failure on NCCTG-N9831. Since we did
not know if it was a real problem or if we just happened to have
a few cases at the same time, we decided to temporarily halt accrual
to the third arm of the trial — AC followed by paclitaxel
and concurrent trastuzumab — until we had more time to do
two things.
First, we had to evaluate the clinical course of those few patients
who developed congestive heart failure. Second, we had to analyze
the data based on all of the more than 700 patients enrolled up
to that point. Eventually, we found that there were just a few
patients who had developed congestive heart failure and that the
patients who developed congestive heart failure had prompt improvements
in their clinical symptoms with medication.
We submitted this information to our independent data monitoring
committee. Since the cases of congestive heart failure were below
the threshold we had established in the protocol in June 2002,
it was recommended that we reopen accrual to this third arm of
the trial. We meet with our cardiologists on a monthly basis to
look at all of the data from this study. We have very good compliance
with the cardiac testing we recommend as part of this clinical
study.
Based on data in the metastatic setting, trastuzumab is associated
with congestive heart failure. In the adjuvant setting, it is going
to be a matter of assuring that the incidence of congestive heart
failure is low and working on potential predictors of congestive
heart failure. There are trials being devised to address this issue.
We are looking at hypertension, the patient’s age and radiation
therapy to the left chest as being predictors of cardiotoxicity.
We are also doing quality control to avoid enhancing the potential
cardiotoxicity of trastuzumab.
Theoretically, it makes sense that trastuzumab will have a role
in the adjuvant setting. But first, we need to finish the clinical
trials to prove that point. Then we will have to look at ways to
ameliorate cardiotoxicity, even if it’s only a few percentage
points.
Ejection fraction assessment in the NCCTG-N9831
adjuvant trastuzumab trial
We perform very thorough analyses of ejection fractions as part
of NCCTGN9831, and we have submitted the data to the ASCO 2003
meeting. The specific data we will present are based on the evaluations
of ejection fraction after AC chemotherapy. We have a lot of clinical
experience with AC, but there’s a scarcity of data regarding
its effect on ejection fraction. We found that AC, at a cumulative
dose of 240 mg/m2, had a zero incidence of congestive heart failure,
but there were decreases in ejection fraction. These decreases
in ejection fraction tended to be transient.
Our opinion is that ejection fraction may be an interesting marker,
but we don’t know if frequent measurements are good in terms
of predicting who will develop congestive heart failure. At this
time, I cannot comment on the effect of trastuzumab on ejection
fraction.
Adjuvant trastuzumab use in and out of the clinical
trial setting
If someone uses trastuzumab outside of the clinical trial setting,
they’re essentially shooting in the dark. We do not yet understand
the duration of therapy, the schedule to be used in combination
with chemotherapy and the potential risks or benefits the patients
may derive.
We have several clinical protocols available. I hope that every
woman diagnosed with breast cancer tells her physician, “If
I have this bad prognosis, I want to participate in the clinical
trial that will help answer the question.”
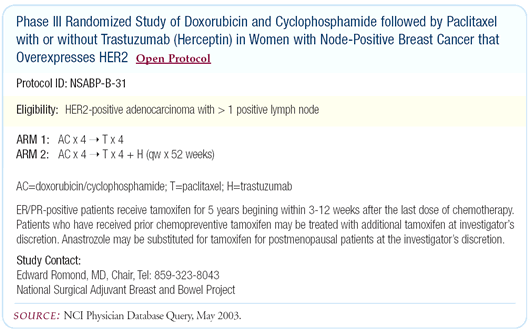
The NSABP is also conducting a very good trial, also based on
solid scientific principles. The NSABP trial has two arms — AC
followed by paclitaxel, and AC followed by paclitaxel concurrent
with trastuzumab for three months, followed by trastuzumab alone.
The NCCTG trial has three arms. NSABP-B-31 is using paclitaxel
once every three weeks, as in CALGB-9344, while N9831 is utilizing
weekly paclitaxel.
Nonprotocol management of patients with node-positive
breast cancer
The management of patients with node-positive breast cancer has
become more complex in the last year, and we now have several very
good regimens. However, we don’t have proof that any one
of these regimens is absolutely better than another. The options
today include the FEC regimen, which is not commonly used in the
United States, TAC regimen and sequential AC followed by paclitaxel
or docetaxel.
If I’m going to use AC followed by a taxane, I tend to use
the dose-dense regimen published in the Journal of Clinical Oncology
based on CALGB-9741, or I may still use AC once every three weeks
followed by weekly paclitaxel. If I were to use docetaxel, then
I would use AC once every three weeks followed by docetaxel once
every three weeks, because of docetaxel’s tolerability when
administered once every three weeks compared to weekly. When I
use the AC every-two-week regimen, I use pegfilgrastim rather than
filgrastim. While we do not have data on that, I believe it is
much more convenient for patients, and we have incorporated it
into our clinical practice.
Select publications
Page 2 of 2
|
