| You
are here: Home: BCU 4|2003: Michael
F Gnant, MD

Edited comments by Dr Gnant
ABCSG-12: Adjuvant anastrozole or tamoxifen
in combination with goserelin (± zoledronic acid) for hormone
receptor-positive, premenopausal breast cancer
Trial background and rationale
We have conducted trials with premenopausal breast cancer patients
with endocrine-responsive disease for more than 10 years, attempting
to optimize treatment, particularly without the use of cytotoxic
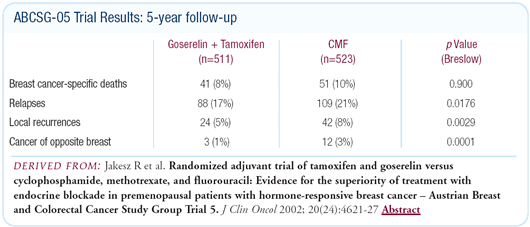
chemotherapy. In ABCSG- 05, we showed that the chemical ovariectomy
with goserelin plus tamoxifen was equivalent or actually better
than the standard CMF. So, in Austria, we have come to a consensus
that this hormonal therapy is appropriate for premenopausal women
with low- and intermediate-risk, hormone-responsive breast cancer.
We feel the effect of cytotoxic chemotherapy in these patients
is more an endocrine effect, and we can show that those patients
who experience
amenorrhea during chemotherapy do much better than those that
don’t. So at least part — maybe 70 or 80 percent — of
the benefit of chemotherapy is actually an endocrine effect, rather
than a direct cytotoxic effect.
This treatment approach is not as popular in the U.S. as it is
in Europe, partially because the history of the endocrine treatment
is not as extensive in the U.S. While medical oncologists in the
U.S. are beginning to prescribe combinations of goserelin with
other agents, cytotoxic chemotherapy remains the standard.
Given the results of ABCSG-05, the next logical step was to determine
how to improve on the combination of goserelin/tamoxifen. It has
been demonstrated several times that the aromatase inhibitors anastrozole
and letrozole decrease serum estradiol levels in women even more
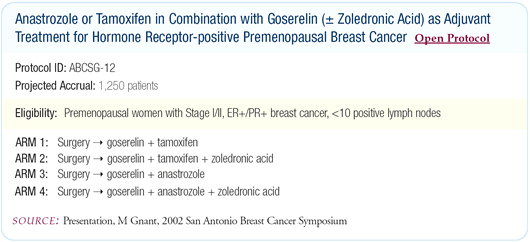
effectively than tamoxifen, so ABCSG-12 was designed to compare
the combination goserelin/tamoxifen to goserelin/anastrozole.
Trial design
The ABCSG-12 trial has four arms comparing goserelin/tamoxifen
to goserelin/anastrozole with or without zoledronic acid. We included
zoledronic acid because it’s the most potent bisphosphonate
pharmacokinetically and we were concerned about the risk of osteoporosis
with the aromatase inhibitors. Chemotherapy is only permitted as
neoadjuvant therapy. No postoperative chemotherapy is allowed.
We did not include a tamoxifen-only arm because we tried to build
upon our own results with goserelin/tamoxifen, which is now a national
standard in Austria. I also believe tamoxifen-only treatment in
premenopausal women is debatable because there is reasonable evidence
you need to include some cytotoxic treatment.
Rationale and dosing for zoledronic acid
We still do not know whether bisphosphonates can impact survival,
but the claim that they reduce bone metastasis is logical. If you
can impact osteoclast function, then you might in some way delay
or inhibit bone metastasis. In addition, zoledronic acid has exhibited
antitumor functions, specifically antiangiogenic and apoptosis-inducing
effects in animal models.
We began our trial with a dose of eight milligrams of the agent
every month, higher than what is used in osteoporosis — hoping
to see a survival benefit. However, alarming information about
renal toxicity with the drug came out after the trial opened, so
we decided to go back to the recommended antiosteoporosis dose.
Although safety is the most important directive you can use as
a study group, this was probably a missed research opportunity.
When we analyzed the serum creatinine levels of the 100 patients
who received the higher dose — and we have more than a thousand
such measurements — there was never even a slight increase
in serum creatinine.
The alarming toxicity data came from heavily pre-treated myeloma
patients — some of whom had impaired renal function before
they ever began zoledronic acid. We are treating younger breast
cancer patients who usually have perfect renal function, so I believe
it would have been a safe approach. Clearly, we had to put safety
first and reduce the dose to four milligrams every six months.
The dose of zoledronic acid used in animal models where antitumor
mechanisms were seen would translate to a 32-milligram dose in
humans, which is currently considered unsafe. However, I have heard
that there are Phase I and II trials in myeloma patients with even
higher doses administered more slowly. It’s believed that
if you increase the infusion time to two or three hours, the kidneys
are pharmacokinetically able to handle the higher doses, but I
haven’t seen any written or published data on that.
Next page
Page 1 of 2
|
