| You
are here: Home: BCU 4|2003: J
Michael Dixon, MD, FRCS

Edited comments by Dr Dixon
Neoadjuvant anastrozole trial
Several years ago, we conducted a trial using three months of
neoadjuvant anastrozole in 23 postmenopausal women with estrogen
receptor-positive breast cancer. Interestingly, 22 of those patients
continued on adjuvant anastrozole after the trial ended. It has
now been four years since these patients started on adjuvant anastrozole,
so we have a reasonable follow-up period from which to gather additional
data.
We evaluated whether the response rate to anastrozole was dependent
on the initial tumor’s HER2 status. It was a small study
with only 23 patients, but we obtained reasonable material from
22. We found that six patients had tumors with a 3+ score for HER2
protein overexpression, and all six responded clinically to anastrozole.
All six of the patients’ tumors decreased in size by more
than 50 percent in bidimensional area, thereby fulfilling the criteria
for a partial response with only a three-month treatment period.
This supports previous evidence that the aromatase inhibitors
are effective for patients with HER2 3+ tumors, and it's the first
data showing that anastrozole is effective in this group. A slightly
lower response rate was seen in patients with tumors that were
not HER2 3+, but there was no statistically significant difference
between response rates. When we looked at the percentage reduction
in tumor volume, there was a trend for the HER2 3+ tumors to shrink
more than the tumors that were HER2-negative. This fits in with
the data suggesting a better response rate with the aromatase inhibitors
in patients with HER2 3+ tumors.
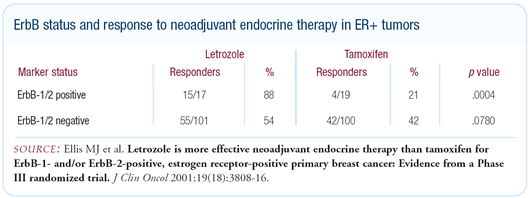
It also contrasts with the data presented by Matt Ellis last
year at San Antonio, in which patients with HER2 3+ tumors did
not have a consistent reduction in proliferation when treated with
neoadjuvant tamoxifen. We, too, have evaluated tamoxifen in the
neoadjuvant setting, and found the same results as Matt Ellis — HER2
3+ tumors don’t have a consistent change in proliferation
with tamoxifen.

All of the tumors — HER2-positive and negative — also
demonstrated a reduction in proliferation over the three-month
neoadjuvant period. The order of reduction in proliferation was
the same for the tumors that were HER2-positive and negative.
Proliferation decreases within a few days of starting anastrozole,
and this opens up a new avenue for treatment. The idea is — if
you see a postmenopausal woman with breast cancer, you don’t
really have to worry about the date of surgery, because you can
put her on anastrozole and know that by the time you operate, her
tumor will be biologically different.
The IMPACT trial of neoadjuvant anastrozole
The neoadjuvant anastrozole study, known as the IMPACT trial,
has finished recruiting 330 patients who were randomized to anastrozole
alone, anastrozole and tamoxifen or tamoxifen alone. From this
study, we should be able to determine whether there are any differences
in response rates to these agents in patients with HER2-positive
tumors. We will also be able to evaluate biological end points,
and that should tell us a little bit more about the interaction
between anastrozole and HER2.
The IMPACT trial will tell us a lot about how these drugs work,
and I think it is a very important study. I’m pleased we
have completed it. The results, however, won’t be available
until the middle of next year.
After surgery, if the patient had responded to the three months
of neoadjuvant endocrine therapy, they continued on the same medication
and remained blinded. There are some patients still on the combination.
If the patient did not respond, then they were unblinded and put
on the other agent.

Biologic effects of the tamoxifen and anastrozole
combination
We don’t know what happens in the tumor when we give both
anastrozole and tamoxifen, but we could guess. I think it will
be like the effects of tamoxifen. Many of us thought that the combination
was never going to work anyway, because when you reduce estrogen
levels with anastrozole, tamoxifen acts like a partial estrogen
agonist. I don’t think there was ever any good scientific
rationale to the combination.
It’s interesting to speculate about what will be seen inside
the tumor with the combination. The good news is that we have sequential
biopsies and we will be able to look at which genes are switched
on or off by the three treatments in the IMPACT trial. That will
give us real insight into how the combination arm works.
Resistance to tamoxifen in patients with HER2-positive
cancer
I think resistance occurs because tamoxifen’s mode of action
is through the HER2 pathway, whereas anastrozole works independently
of HER2. That may be too simple. In the series of patients on the
IMPACT trial, and others we’re treating with aromatase inhibitors,
we will be able to look at more details, because we have fresh
tissue before diagnosis, during treatment and after treatment.
I’m using micro-array techniques and proteomics, and we
are about to see — in great detail — how these drugs
interact. The simplistic way we now look at how these drugs work
will be overshadowed by what we learn from these studies.
Selection of patients for neoadjuvant endocrine
therapy
Patients who express the most estrogen receptor (ER) will have
the greatest reductions in tumor volume with neoadjuvant endocrine
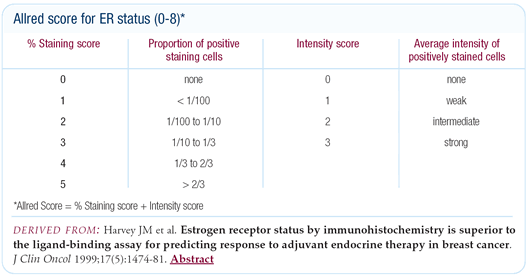
therapy. We only treat patients with Allred scores of 6, 7 or 8.
The Allred score is a composite of the percentage of cells that
stained and the intensity of their staining. The percentage of
cells staining is classified from 0 through 5, and the intensity
of cells staining is rated as 1, 2 or 3. Then if you add, for example,
5 and 3 together, you have an Allred score of 8. In order to initiate
therapy, the cutoff we use for positivity would be over one-third
of the cells staining strongly or over two-thirds staining moderately.
Fortunately, the majority of postmenopausal women are strongly
ER-positive, and these are the women most likely to benefit. Their
median reduction in tumor volume with three months of neoadjuvant
anastrozole therapy is over 80 percent. Not only does a large part
of the tumor disappear within that three-month period, but the
nature of the tumor also changes — there is reduced cellularity
and proliferation.
If you select patients for treatment carefully, the response
rates to neoadjuvant endocrine therapy are very high. In a poster
we presented at the San Antonio Breast Cancer Symposium, the response
rate to anastrozole in that group of patients was 80 percent and
they had a greater than 50 percent reduction in a bidimensional
area with three months of neoadjuvant therapy. We were able to
convert two-thirds of the patients requiring mastectomy to breast-conserving
surgery.
Underutilization of neoadjuvant endocrine therapy
I believe neoadjuvant endocrine therapy is underutilized. It
is valuable in some patients, particularly the elderly, and the
biggest increase in breast cancer incidence over the next decade
will be in older patients. Currently, 40 percent of women with
breast cancer are over 70 years of age, and that is likely to go
up to nearly 50 percent over the next decade. These are women whom
you wouldn’t necessarily want to give neoadjuvant chemotherapy.
We have very good drugs, such as the aromatase inhibitors, that
produce consistently high response rates and reductions in tumor
volume.
Next page
Page 1 of 2
|
