| You
are here: Home: BCU 4|2003: Michael
F Gnant, MD

Interim bone mineral density results
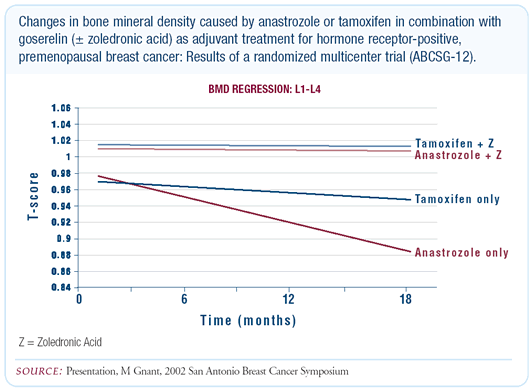
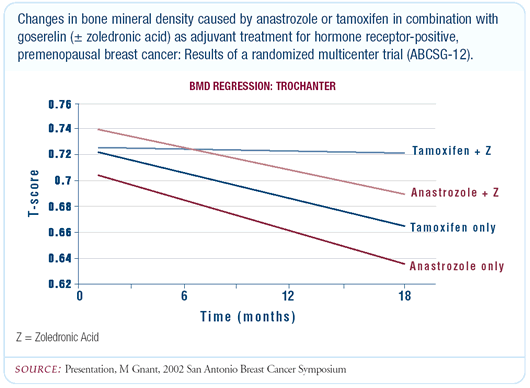
The early results of ABCSG-12 demonstrate that the combination
of goserelin/anastrozole, and goserelin/tamoxifen to a lesser degree,
leads to significant deterioration in bone mineral density in premenopausal
women and that this can be completely counteracted by zoledronic
acid. Even though tamoxifen has an agonistic effect on bone, when
combined with the more potent agent, goserelin, it results in a
net reduction in bone density. The bone deterioration is more pronounced
with anastrozole/goserelin, but there is not a significant difference
at this time. The main message is that zoledronic acid was able
to completely prevent bone loss, regardless of which hormone combination
the patients received.
While the trial is ongoing, we decided we needed to present the
bone mineral density results after the interim analysis. We wanted
to inform physicians and patients about the effects on bone and
give them the opportunity to do something to counteract these,
if necessary.
The decrease in bone mineral density is about 10 percent — osteopenic
rather than osteoporotic — so treatment is not mandatory.
However, patients can take precautions such as exercise and vitamin
substitution.
Also, we are observing a strong correlation between age, baseline
bone mineral density and changes in bone mineral density. In younger
patients, if you decrease the estradiol with goserelin/anastrozole
to almost undetectable levels, they suffer a more pronounced deterioration
in bone mineral density than the perimenopausal patients. Younger
patients on anastrozole or tamoxifen may be at higher risk, although
we have never seen a patient with a T-score change of more than
minus 2.5, in whom bisphosphonate treatment would be mandatory.
Anticipated long-term results
One problem with this study is that the event rate is lower than
we expected. In 15 years of designing and conducting clinical trials,
it has always been my experience that we overestimate the event
rate. This is good for patients, but not for the trial. I expect
that next year we’ll have to consider increasing the sample
size, basing it on the actual event rate in the first two years,
rather than on our pretrial projections.
I suspect that we will not see a survival benefit for the bisphosphonates
at our current dose of zoledronic acid; however, we may see a slight
survival advantage for the anastrozole combination, based on the
ATAC data. Goserelin renders all the patients postmenopausal, and
I don’t know of any reason women who become postmenopausal
as a result of therapy would respond differently than those who
become postmenopausal naturally.
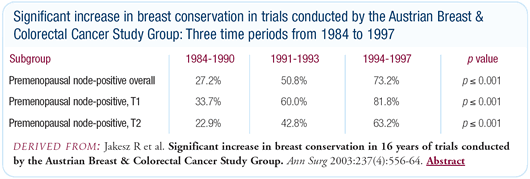
Breast-conserving surgery
At our institution, we have a 75 to 80 percent breast conservation
rate. In reviewing six of our clinical trials, we found the rate
was as high as 82 percent in premenopausal patients with T1 tumors.
With extensive use of preoperative chemotherapy and improved operative
techniques, I believe mastectomy should become an obsolete surgical
procedure.
Significant variance exists in breast conservation rates from
country to country. Some of that is a cultural difference in the
surgeon’s approach to patients, and some of that variance
has to do with the availability of radiotherapy resources. In countries
outside the western world, this is a major problem.
From the patient’s point of view, it is very important
that we have the resources available to facilitate breast conservation
everywhere in the world. In the year 2003, I feel it’s unacceptable
for any patient not to be offered a reasonable choice of organ
conservation in all surgical oncology indications, whether it's
breast, rectal or other cancers.
Neoadjuvant hormonal therapy and disease progression
I do not routinely use preoperative hormonal therapy outside
of clinical trials. To me, the problem with the neoadjuvant endocrine
treatment is that you have a certain rate of nonresponders, which
we don’t have with cytotoxic chemotherapy. For example, in
our neoadjuvant trial, ABCSG-14, comparing three versus six cycles
of epirubicin and docetaxel, we have a 70 to 80 percent response
rate, probably a 15 to 25 percent complete pathological remission
rate, and no progressive disease.
Disease progression during neoadjuvant therapy is a big problem.
Patients are eager to have their lump removed, and while they may
be willing to undergo neoadjuvant therapy to increase their likelihood
of breast conservation, if you use endocrine therapy you have to
tell them there’s a 10 percent chance the lump will actually
grow. I believe we should continue to test this therapy in clinical
trials on cohorts of patients not suitable for cytotoxic chemotherapy.
Sentinel node: A standard of practice
We are not involved in sentinel node trials because this procedure
is already the standard of care in Austria. One of the advantages
of being in a small country with a well-functioning network of
breast centers is that you can quickly translate an experimental
procedure into daily practice. A set of guidelines was established,
teaching courses were offered, institutions exchanged surgeons
and sentinel node biopsy became a standard in the country.
Research in radiotherapy
We are conducting a trial randomizing patients with low risk
after breast conservation to radiation or no radiation. Patients
must be over age 60, nodenegative, on endocrine therapy and have
a tumor size less than 3 centimeters. We have shown in retrospective
studies that these patients have a local recurrence rate of only
about two percent, so we need to determine if there’s a subset
of patients who do not need adjuvant radiation after breast conservation.
This study will require a large number of patients and a long follow-up
to determine equivalence of these two approaches.
We also have two Austrian institutions exploring intraoperative
radiotherapy (IRT). It’s very compelling to substitute this
for five weeks of treatment, but from what I know about the concept
of fractions, the rationale for this approach may be debatable.
Also, in all the trials that I’m aware of, IRT is being used
in patients at very low risk, and these are patients who probably
do not need radiation whatsoever.
Future of research in breast cancer treatment
and prevention
I believe future research will focus on defining subgroups of
women with lowand intermediate-risk disease and finding appropriate
treatments. One of the problems at this point is that progress
may be made in smaller increments and may cost more. It may be
easier and cheaper to increase an effect from 50 to 70 percent,
than to increase it from 70 to 75 percent.
In the population at high risk, efforts to maximize dosing and
then rescue patients, as with stem cell transplantation, have failed
overall. It's probably a qualitative effect, rather than a quantitative
effect, in that tumor cells vary in their response to different
treatments. I believe researching areas such as growth factors
and tyrosine kinase treatment will be important.
Apromising trend in research is the emerging chip technology.
This allows researchers to target specific mutations present in
each cancer, which will hopefully lead to the development of tailored,
more effective treatments, especially in the population at high
risk. Chip technology may also be used in prevention by helping
us understand the transition from atypical ductal hyperplasia to
cancer, how invasion occurs and determining which women — other
than BRCA carriers — are at high-risk for developing breast
cancer. Once we identify these patients, we can intervene with
endocrine treatment for prevention.
Patient benefits from participation in clinical
trials
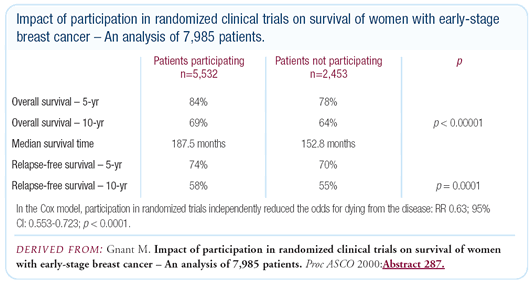
We have demonstrated that patients gain individual benefits from
participating in clinical research trials. We did a retrospective
analysis, presented at ASCO in 2000, comparing 5,700 patients in
clinical trials with 2,000 patients with similar risk treated by
a so-called standard treatment. These were all primary breast cancer
patients and there was almost a 10 percent survival difference
in favor of the patients on trials.
Clearly there is a problem with the standard of treatment patients
receive outside clinical trials. In our analysis, we were able
to look at the treatment received by the patients not in trials,
and about 90 percent of these patients received therapies we would
consider suboptimal. Clinical trials are designed to ensure that
patients receive optimal therapy. I see 500 breast cancer patients
a year, and by treating patients within a clinical trial, I have
all kinds of assistance — checklists, monitors, data verifications — to
prevent me from forgetting something.
An additional explanation for the survival benefit of clinical
trial participation is that, through required visits and tests,
we pick up other medical problems that can be remedied. While a
patient’s risk reduction is about 40 percent after ten years,
only 20 percent comes from breast cancer-related survival. The
other 20 percent is related to other causes of death. I know a
prospective randomized comparison of regular follow-up versus symptom-oriented
follow-up doesn’t show any survival difference, but personally
I don’t agree with that data.
When we treat patients within clinical trials, we not only help
that individual patient, but we also help the next generation of
patients and I believe that this is the most important task right
now.
Select publications
Page 2 of 2
|
