| You
are here: Home: BCU 4|2003: J
Michael Dixon, MD, FRCS

Selecting an agent for neoadjuvant endocrine
therapy
The problem with putting a newly diagnosed patient on tamoxifen
is that it takes two to three weeks for the levels to accumulate
in the blood. Additionally, the rate of deep venous thrombosis
(DVT) and pulmonary embolus increases immediately before surgery.
For these reasons, I do not find preoperative tamoxifen particularly
attractive. Anastrozole doesn’t appear to increase the risk
of DVT or pulmonary embolus; hence, it is a more attractive agent
to use before surgery.
Preoperative anastrozole is also more appealing because it affects
the basic biology of the tumor. There’s always been a fear
that surgery might spread breast cancer. I believe this is theoretical,
rather than practical. Nonetheless, the cancer cells are less likely
to implant if one operates on a tumor under the influence of a
drug that turns off proliferation.
Predicting the efficacy of neoadjuvant endocrine
therapy
I believe that over the next several years, the neoadjuvant model,
by which one can access the tumor on numerous occasions during
the three months of therapy, will provide very valuable data about
how these drugs work. We also hope to be able to identify — within
a few days of starting a drug — whether the patients will
derive a long-term beneficial response.
Within 24 hours of starting these drugs, changes occur within
the tumor. The aim of our new work is to develop a series of markers
to allow us to predict within two weeks of starting a drug, such
as anastrozole, whether the patient will derive long-term benefit.
We’re able to correlate these changes at two weeks with the
response at three months.
Eventually this might allow us to diagnose a patient, start them
on a drug and operate on them. Then, by looking at the tumor at
the time of surgery, we may be able to determine whether that drug
had the expected effect and should be continued long-term. Our
hope is to develop some simple tests to allow us to look at an
individual patient and say, “Yes, this patient should be
treated with this drug,” or “This patient had the changes
that we would hope would predict long-term benefit.”
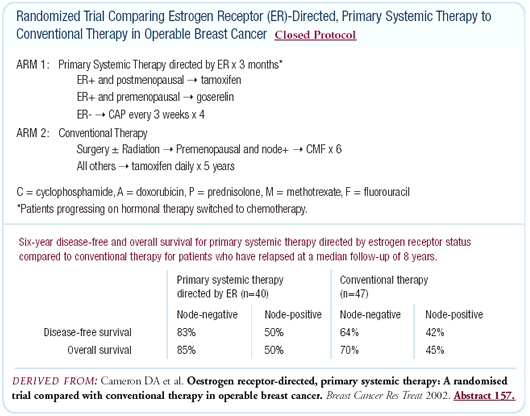
Estrogen receptor-directed, primary systemic
therapy compared to conventional therapy in operable breast cancer
At the San Antonio Breast Cancer Symposium, we presented long-term
followup data on patients randomized to conventional therapy (mastectomy
followed by appropriate adjuvant therapy) or neoadjuvant therapy
selected on the basis of the patients’ estrogen receptor
status. Premenopausal patients with estrogen receptor-positive
cancers were randomized to neoadjuvant therapy with goserelin.
Most postmenopausal women with estrogen receptor-positive cancers
were randomized to neoadjuvant therapy with tamoxifen, although
some received aromatase inhibitors.
This trial, which involved a relatively small number of patients,
offered no evidence that patients receiving neoadjuvant therapy
did worse and a slight suggestion that they actually did better.
There was no difference between the patients who received neoadjuvant
endocrine therapy and neoadjuvant chemotherapy. Although the number
of patients involved is not sufficient for us to draw any definite
conclusions, it's an interesting study.
Integrating adjuvant anastrozole into clinical
practice
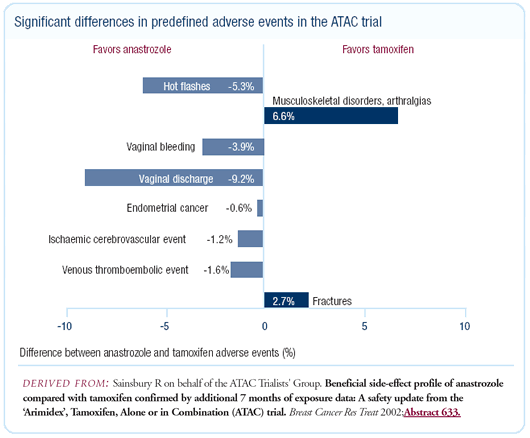
The ATAC data are very impressive for adjuvant anastrozole. To
some extent, I think we expected the separation in the curves to
increase, as they did. The bone data was a concern but I don’t
think it will be too much of an issue because studies show that
use of bisphosphonates can avoid this problem.
The reduction in vaginal bleeding, vaginal discharge, hot flushes
and endometrial cancer associated with anastrozole was much more
impressive. I believe that the overall benefits are much greater
with aromatase inhibitors in postmenopausal women with estrogen
receptor-positive tumors.
If one looks at the differences between an anthracycline-containing
regimen and CMF, the benefits are quite modest. In the ATAC trial,
the benefits are actually greater, yet, throughout the world, anthracyclines
are now first-line therapy for patients with breast cancer. We’ve
not yet jumped to using an aromatase inhibitor as first-line therapy;
however, I don’t think this is very far off.
Interchangeability of the aromatase inhibitors
in the adjuvant setting
There are no data for letrozole or exemestane in the adjuvant
setting. Anastrozole is the only drug that’s been tested
in that setting, and I believe it is the drug we should use.
Each of the aromatase inhibitors is slightly different, and they
have slightly different effects on circulating estrogen levels.
Exemestane may have some androgenic activity, which may have some
beneficial effects, but has some negative effects as well. It may
have some better bone effects, but it may cause a bit more weight
gain. We don’t know at the moment.
We probably need some direct comparative data of the side-effect
profiles of the different drugs. I suspect it might come down to
which is the most tolerable, since they’re all effective.
Anastrozole has a head start, because it has a better side-effect
profile than tamoxifen, and we always thought tamoxifen was a pretty
safe drug. Until we have data comparing the different drugs, we
have to use the drug that has been tested in this setting.
Tolerability of anastrozole versus tamoxifen
Tolerability of anastrozole is excellent in the group of patients
we’ve treated, who tend to be a bit older. The patients come
in and say, “How do I know I’m on a drug, because I
don’t feel any different? I don’t have any side effects.” Vasomotor
symptoms are a real problem for women taking tamoxifen. A number
of our patients have had to stop tamoxifen, because their quality
of life was so poor. The long-term prognosis is excellent for many
women on adjuvant hormonal therapy; therefore, it’s not a
great idea to give them a drug that makes them feel worse.
With tamoxifen, some women are disabled by vaginal discharge.
This is particularly true of women with any degree of prolapse,
who have a constant leak. For a few women, it affects their quality
of life to a major degree. In the metastatic setting, there was
virtually no vaginal discharge associated with the aromatase inhibitors,
and it has not been a problem in the adjuvant setting.

A large percentage of women on tamoxifen complain of weight gain,
while anastrozole doesn’t seem to cause weight gain. The
art of medicine is to find agents that suit the patient and minimize
the side effects. Anastrozole offers us another option.
IBIS-II trial
IBIS-II, a prevention trial, will compare anastrozole to placebo
in women at high risk of developing breast cancer. In the UK, tamoxifen
as prevention has not caught on because it has a high rate of morbidity.
The IBIS-I study showed a very minimal effect for tamoxifen with
considerable morbidity. Anastrozole looks like a better agent for
prevention than tamoxifen, so I agree with the direct comparison
to placebo.
Based on the ATAC trial data, I would expect anastrozole to dramatically
decrease the number of breast cancers that develop. I think anastrozole
should be superior to tamoxifen in that setting.
An IBIS-II subprotocol will also evaluate the effects of anastrozole
on bone density. The trial is randomizing patients into three groups:
(1) high risk for osteoporosis (evidence of osteopenia on DEXA
scans), (2) intermediate risk for osteoporosis, and (3) low risk
for osteoporosis (bones are very dense). The patients at high risk
will receive bisphosphonates, the patients at intermediate risk
will be randomized to bisphosphonates and the patients at low risk
will receive anastrozole alone without being randomized to bisphosphonates.

Select publications
Page 2 of 2
|
