| You
are here: Home: BCU 1|2003: Gabriel
N Hortobagyi, MD
Edited comments by Dr Hortobagyi
Implications of the ATAC trial in clinical practice
The results of the ATAC trial are quite compelling. Even if you
assume for the sake of argument that the curves will come together
with further followup, the safety profile of anastrozole is still
clearly better than tamoxifen. I cannot prevent endometrial cancer
short of removing the uterus, but I can prevent or treat osteoporosis
and fractures. Since the safety profile of anastrozole is better
than tamoxifen and it is therapeutically superior, I have a problem
not offering anastrozole to my postmenopausal patients — not
as a neutral choice but as a better choice. I do discuss with my
patients the enormous amount of clinical experience we have with
tamoxifen, but if my sister developed breast cancer today, I would
certainly recommend anastrozole as opposed to tamoxifen.

| Updated
47-month follow-up of the ATAC trial |
“With
increased follow-up, AN continues to show superior efficacy
to TAM, these benefits being most apparent in the clinically
relevant hormone receptor-positive population. These results
confirm that the benefits observed with AN are likely to be
maintained over the long-term. A safety update has confirmed
the findings of the main analysis…” |
| SOURCE:
Buzdar A et al. The ATAC ('Arimidex', Tamoxifen, Alone
or in Combination) trial in postmenopausal women with early
breast cancer — Updated efficacy results based on a median
follow-up of 47 months. Breast Cancer Res Treat 2002;
Abstract
13. |

Use of other aromatase inhibitors in the adjuvant
setting
I do not use the other aromatase inhibitors in the adjuvant setting,
because there are no adjuvant data. While we have to extrapolate
in a number of situations, I do not see an advantage for the other
aromatase inhibitors from the existing data. It is possible that
some time in the future, someone will show a distinct advantage
of one of these other agents, but at this point, the data were generated
with anastrozole, so I use anastrozole.
Making clinical decisions in the face of uncertainty
Oncology is one of the classic specialties in which uncertainty
is a way of life because of progress and constant change. It is
important for our professional organizations to have consensus at
various points in the evolution of a particular treatment. Having
said that, those guidelines and consensus statements tend to be
relatively conservative. Most of the time that is perfectly appropriate,
but physicians will have to make individual decisions based on interactions
with patients. Some of those decisions will follow guidelines, while
others will not.
There are situations where departing from a guideline is clearly
wrong. For instance, there is widespread acceptance that aromatase
inhibitors should not be used in premenopausal women. Departing
from the guidelines in that setting is clearly inappropriate because
you would actually reject scientific evidence as the basis for your
decisions. But, in situations where there are data and evidence
to support various options, there is nothing wrong with deviating
from a consensus statement as long as it is appropriate for that
specific patient.
Recommending adjuvant anastrozole based on early
trial results
There is no comparable trial in the history of medical oncology
or breast cancer, and there is no other tumor type with so many
well-planned clinical trials conducted. We are in a leadership position
in oncology, and we can’t advocate doing the best trials and
then ignore the results of those trials. Every single trial we do
brings with it some of the unknown.
We started to move over to tamoxifen well before we had five-year
follow-up. I remember when Michael Baum presented the early data
from the NATO trial in 1982. It had less than two years of follow-up,
and he was already publicly talking about the advantages of adjuvant
tamoxifen — and the NATO trial pales in size and design in
comparison to the ATAC trial.
We have very compelling data about anastrozole from the ATAC trial,
in terms of its therapeutic and safety profile superiority. I would
be doing a disservice to my patients who are candidates for adjuvant
aromatase inhibitor therapy by not presenting the data. I also present
tamoxifen as an option, but in the last six months I would say that
60 percent of my postmenopausal patients chose anastrozole rather
than tamoxifen. There is no right or wrong decision, but for me,
there are compelling data to prefer anastrozole.
Incorporating early research results into practice
I was actually one of the individuals who initially fought against
the use of adjuvant tamoxifen — especially in premenopausal
women — in the 1980s. Up until the early 1990s, in our own
clinical trials at MD Anderson, we did not include endocrine therapy
in the adjuvant treatment of premenopausal women. We learn from
history that we probably fail to understand the impact of emerging
data on the lives of women. Coming from that background, my flexibility
in accepting the new and relatively early data of the ATAC trial
is more significant to me. If I had understood the deep implications
of what tamoxifen could do in terms of saving lives in the early
1980s, I could probably have modified many of our own activities
and policies during the subsequent ten years.
There are situations in which one needs to be much more conservative.
I was much more cautious in the high-dose chemotherapy area, because
there was much to lose. However, the safety issues of high-dose
chemotherapy in the 1980s and of aromatase inhibitors in the 21st
century are so enormously different that we cannot even compare
them.
Providing patients with treatment options and
recommendations
One of our major goals is to fully educate our patients by giving
them relevant, accurate and complete information, so that they understand
their prognosis, treatment options and the benefit-to-risk ratio
they will face with each of those options. But we can’t stop
there. We also need to make a recommendation after that education.
Obviously this recommendation will incorporate our biases and prejudices,
but we are better qualified — even with those biases and prejudices
— than a patient who just had “oncology 101” during
the previous 20-30 minutes. I feel very strongly about that.
Over the past 30 years in medicine, we have moved from a paternalistic
approach to the other extreme. Many of my colleagues try to be so
neutral that they do not make a recommendation. The burden of decision-making
has been removed completely from the physician, who is best qualified
to make that choice or recommendation, to the patient, who sometimes
is — but most of the times is not — in the best position
to make that choice without guidance. I understand and agree that
patients need to have autonomy. We clearly have the obligation to
inform them fully, but I think we need to go beyond that. We have
to get to know our patients and understand their motivations, their
understanding of risks and benefits, their definition of therapeutic
gain and their acceptable level of risks and side effects. As physicians,
we need to help them make a decision. To abrogate that responsibility
is an unfortunate — and I hope temporary — trend in
the medical profession.
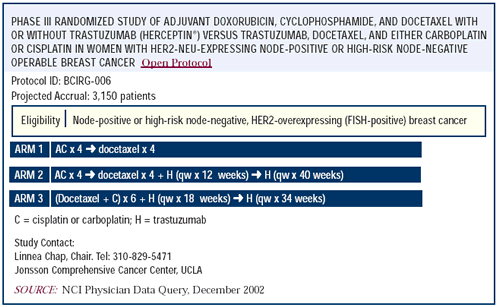
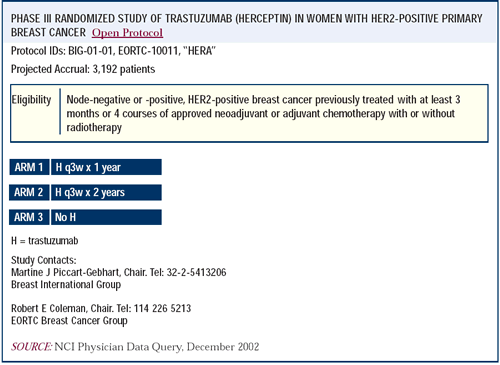
Adjuvant randomized clinical trials of trastuzumab
There is a substantial body of data suggesting that while there
is a slight excess in cardiac events with trastuzumab and anthracycline-based
chemotherapy in the adjuvant setting, it is unlikely to affect survival
in any major way. We have elected to support the BCIRG adjuvant
trial, but the NCCTG and NSABP trials are equally worthwhile. All
three will contribute to our understanding of how best to incorporate
trastuzumab in the adjuvant setting. While it is reasonable to ask
whether we can derive the same amount of benefit from trastuzumab
with a non-anthracycline-containing regimen as with an anthracycline-containing
regimen, the bulk of the data from retrospective analyses of many
of our previous trials points to the importance of anthracyclines
precisely in HER2-positive patients.
I think it is shortsighted to abandon anthracyclines on the basis
of a potential risk for toxicity. The history of oncology is full
of toxic agents that were almost discarded until someone found a
way to administer them safely. That is true for anthracyclines,
cisplatin, alkylating agents and taxanes. We should not be surprised
that these drugs have toxicity, but we should not discard them lightly.
We should learn to use them safely through clinical trials, and
there are many ways to address this issue of developing the safest
and most effective combination with trastuzumab.



Dosing and scheduling of chemotherapy
The expression “where there’s smoke, there’s
fire” applies to an issue we have been studying for decades
— chemotherapy dose and schedule. We learned the hard way
with high-dose chemotherapy and bone marrow transplantation, but
I think there is room to test various parts of that hypothesis.
Several interesting trials are exploring the question of dose-density
versus dose-escalation or a bolus of single agents versus combination
chemotherapy.
Metronomic chemotherapy was resuscitated in the process of developing
angiogenesis inhibitors. Anumber of investigators found that fairly
traditional chemotherapeutic agents have an antiangiogenic effect
and substantial antitumor activity when given chronically in low
doses as opposed to intermittently at high doses.
The experience with taxanes and fluoropyrimidines highlights the
importance of scheduling. It is quite likely that the doses and
schedules initially approved for both taxanes might not be optimal,
and there may be other less accepted or unexplored schedules that
might lead us to better administration of these drugs.
Capecitabine: A targeted chemotherapy
Capecitabine is a fascinating agent, which in addition to teaching
us more about the fluoropyrimidines in general, brought out the
targeted aspect of chemotherapy. Conceptually, capecitabine is a
hybrid of a traditional cytotoxic agent and a targeted agent activated
on site. This is an absolutely fascinating observation, not dissimilar
to the aromatase inhibitors, which also utilize the mechanism of
targeting an area rich in the enzyme relevant to the intervention.
We have a lot more to explore in this area.
| Enzymatic
activation of capecitabine |
"Capecitabine
is not intrinsically cytotoxic, and requires conversion to
5-FU via a three-step enzymatic cascade . . . The final conversion
step, which results in the generation of 5-FU, is mediated
by thymidine phosphorylase (TP), an enzyme with significantly
higher activity in tumor tissue than normal tissue . . . TP
expression correlates with fast malignant growth, aggressive
invasion potential, and poor patient prognosis. TP activation
may, therefore, enable capecitabine to specifically target
aggressive cells. In addition, the crucial role of TP in the
activation of capecitabine provides a clear rationale for
combining capecitabine with other antitumor agents that further
upregulate TP in tumor tissue..." |
| EXCERPTED
FROM: Seidman AD, Aapro M. Oncologist 2002;7(Suppl 6):1-3. |
Neoadjuvant trial of capecitabine-docetaxel
As a group, we reached the consensus that for patients whom we
are reasonably certain will receive chemotherapy, we prefer to administer
all chemotherapy before surgery. In a recent neoadjuvant study,
we found that 12 cycles of weekly paclitaxel were better than four
cycles of three-weekly paclitaxel followed by four cycles of FAC.

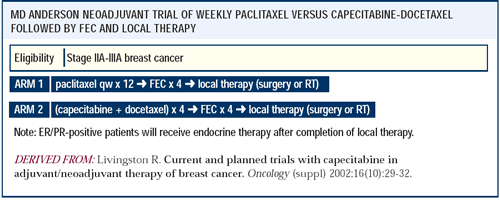
We recently activated a trial of neoadjuvant docetaxel and capecitabine.
This trial will compare four cycles of the capecitabine-docetaxel
regimen to 12 weekly cycles of paclitaxel, with both arms then receiving
four cycles of FEC. We feel that we are building upon the best arm
of a previous regimen while also exploring the interaction between
capecitabine and docetaxel.
Neoadjuvant chemotherapy in women who may be nodenegative
I am comfortable offering neoadjuvant chemotherapy to women who
may be node-negative, because in the past 30 years we have learned
that most things in breast cancer are not black and white, but rather
gradations or trends. Nothing at this point tells me that node-negative
breast cancer is different from node-positive breast cancer with
one positive node. If it is appropriate to use a taxane in the adjuvant
setting for a 2.5 cm breast cancer with a single positive node containing
a 7 mm metastatic deposit, I don’t see a major biological
difference for that same primary without that metastatic deposit
in a single node.
I probably would not use a taxane off-protocol in a patient with
a 1.2 cm node-negative primary, but this too is an evolving area.
The initial trials of taxanes were done in node-positive breast
cancer, but we are in the process of testing them in node-negative
disease. The question will be how to select those patients who should
receive everything, those who shouldn’t receive anything,
and how to define the grades in between.
For the trials we are conducting now, we do fine needle aspiration
of palpable nodes prior to preoperative chemotherapy, so that we
know if there is a node containing malignant cells. But, if I have
a patient with a welldefined 3 cm breast cancer, I'm going to give
chemotherapy whether she has positive nodes or not. Her risk of
recurrence is very similar to that of someone with node-positive
breast cancer.
Chemotherapy in premenopausal women: Benefits
of ovarian ablation
Ovarian ablation with chemotherapy is an area we have not explored
adequately. It is certainly apparent that for premenopausal, estrogen
receptorpositive patients, ovarian suppression is beneficial. The
evolving LHRH analog data suggest that ovarian suppression or ablation
need not be permanent. Even temporary suppression has a substantial
therapeutic benefit, although we do not know the optimal duration
of suppression.
If we accept that this is the case, it is important to develop
cytotoxic regimens that do not cause permanent ovarian ablation.
Since we backed off six cycles of cyclophosphamide to four cycles
of FEC plus a similar duration of a taxane, our preliminary observation
is that fewer patients undergo permanent cessation of menses. So,
the incorporation of a taxane might have other circumstantial benefits
in terms of fertility as well as the major therapeutic goal.
Evolution of breast cancer treatment
Those who don’t know history are condemned to repeat it.
For example, it is fascinating to see the evolution of the St Gallen
consensus statements over time. At one point, we essentially said
that node-negative patients should not receive adjuvant systemic
therapy, but for the most recent one, we did not exclude anyone
with invasive breast cancer. Our interventions haven’t changed
much during that time, but what has evolved is our understanding
of the risks and benefits of treatment and what our patients are
willing to accept and, in fact, request. All of this is in constant
evolution, and what is absolutely true today may not be absolute
tomorrow, and what is totally contraindicated today might become
standard of care in just a few years.
Select publications
|
