|
You
are here: Home: BCU 1|2003: Editor's
office
 |
Editor’s Note |
|
| The Process Works |
“CALGB 9741 is a clear example of the process working.
You have a theoretical idea; you generate laboratory experiments;
you generate clinical experiments and then you obtain buy-in
from clinical investigators to test the idea. In fact, this
is the first major cooperative group randomized trial chaired
by a communitybased oncologist — Marc Citron. The entire
scientific process had buy-in across the board, and it showed
that the system works. In many ways, the presentation of the
data and the publication of the paper are just the beginning.
We have to see how the clinical and research communities react
to the data. But when you open up the pages and you see who’s
alive and who’s dead, and see that there are women who
are alive because of this process who otherwise would have
died, it makes me glad I'm in this field. It's a very exciting,
gratifying result.”
— Larry Norton, MD |
Every September, breast cancer aficionados — hoping to obtain
an early peek at the next “hot story” in clinical research
— eagerly anticipate the arrival of the San Antonio Breast
Cancer Symposium agenda. By the time my copy appeared, Rick Kaderman,
our vice president of Scientific Affairs, had already placed a very
prominent highlight mark on the session scheduled for 9:00 AM, Thursday,
December 12. The profoundly intriguing title of the presentation
to be delivered by Marc Citron was “Superiority of dose-dense
over conventional scheduling and equivalence of sequential vs. combination
adjuvant chemotherapy for node-positive breast cancer (CALGB 9741,
INT C9741).”
Like Rick, I was very eager to learn more about the results of
this study, but I was also well aware that the abstract for this
tantalizing report would not be available until shortly before the
meeting. In late October, I ran into Cliff Hudis, one of the authors
of the paper and a frequent guest on our series. I thought this
might be my opportunity to obtain an inside track on some of the
details of the data. However, no amount of cajoling would loosen
Cliff’s lips. How much of an advantage would the dose-dense
approach convey? Would there be an overall survival benefit? As
I pondered these and other questions, my curiosity was piqued even
more by Cliff’s broad smile and strong encouragement to attend
the session. I sensed that this might be the chemotherapeutic equivalent
to last year’s ATAC trial results, which permanently changed
the adjuvant endocrine therapy landscape.
Knowing that any discussion with CALGB investigators would be embargoed
until the abstract was posted on the San Antonio website shortly
before the meeting, I arranged a November interview with the individual
whose perspective on this trial most aroused my interest. Larry
Norton has spent the last 25 years espousing a mathematical approach
to the war on cancer, and his fervor and commitment to the Norton-Simon
hypothesis has always evoked my admiration, particularly since,
until now, there has been little phase III clinical trial confirmation
of this principle.
In a 1994 interview for this series, Larry predicted that his
mathematical model would someday be tested in a large-scale, randomized
breast cancer trial. It was quite clear at that point that he expected
the results to confirm his long-held speculation that inhibiting
tumor regrowth between chemotherapy treatment cycles played a key
role in the effectiveness of a chemotherapeutic regimen. CALGB 9741
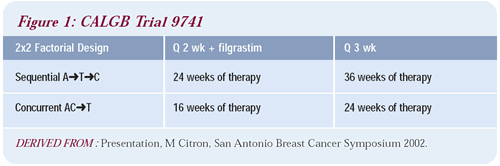
was launched in 1997. The trial had a crisp, highly targeted, two-by-two
factorial design (Figure 1) and because the agents and doses in
the three arms were identical, the study addressed Norton’s
dose-dense theory head on.
I was a bit nervous when I arrived at Larry’s cozy corner
office at Memorial Sloan Kettering on November 18, as I was not
100 percent certain that the abstract had already been released.
We both immediately logged on to the San Antonio website and, thankfully,
the abstract was there in all its glory — a 26 percent relative
reduction in relapse rate and 31 percent relative reduction in mortality
with the dose-dense strategy. These findings were particularly noteworthy
because of the reduced toxicity in these randomization arms. While
Larry quipped “This is one of the first regimens where there’s
nothing not to love about it, “ he was also typically cautious
in predicting how the data would impact patient care and the design
of future clinical trials.
A few weeks later, Marc Citron presented these historic findings
to a packed San Antonio lecture hall. I interviewed Dr Citron later
that day, and his thoughtful views will be presented in our next
issue of Breast Cancer Update. As part of our annual San Antonio
Breast Cancer Symposium “interview blitz,” I also chatted
with 14 other researchers at all hours of the day and night during
the meeting. Like last year’s ATAC extravaganza, there were
a multitude of opinions. However, there was a uniform perspective
among these researchers that the CALGB findings provide important
proof of a principle that, at the very least, will now require routine
discussion of the results with patients contemplating adjuvant chemotherapy.
One of the most rewarding aspects of producing the Breast Cancer
Update series has been the opportunity to participate in documenting
the evolution of clinical research. While large phase III adjuvant
trials like CALGB 9741 and ATAC have been at the focal point of
progress in reducing breast cancer mortality, these studies have,
at times, also been quite maddening in their very gradual evolution.
When I interviewed Michael Baum last year about ATAC, he commented
on the frequent “periods of uncertainty in the evolution of
science and medicine.” Many of the questions posed by investigators
last year about ATAC were answered at this year’s meeting,
where 14 more months of follow-up demonstrated that the disease-free
survival curves of the anastrozole and tamoxifen arms are continuing
to diverge Figure 2). Most of the researchers I interviewed now
agree with the viewpoint Gabe Hortobagyi has been expressing since
the first presentation of ATAC — anastrozole is a rational,
and in many cases, preferable endocrine approach for postmenopausal
women with ER-positive breast cancer. It will be interesting to
see whether clinicians repeat the ATAC experience and have a similarly
cautious initial response to the CALGB data, particularly since
a survival benefit is being reported with the dose-dense strategy.
Twenty-five years ago, a group of 100 researchers gathered for
the first San Antonio Breast Cancer Symposium. That same year, at
the National Cancer Institute, the remitting course of a patient
with Hodgkin’s disease treated with MOPP caused a light bulb
to go off” in Larry Norton’s head. The carefully planned
scientific process that followed this observation culminated in
a startling presentation that has not only provided a new treatment
option for women, but has also confirmed that the clinical trial
process truly works.
—Neil Love, MD

|
|
