| You
are here: Home: BCU 1|2003: Nancy
Davidson, MD
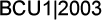
Edited comments by Dr Davidson
Postmastectomy radiation therapy in women with
one to three positive nodes
I usually have my node-positive, post-mastectomy patients evaluated
by a radiation oncologist to discuss the pros and cons of radiation.
If they have four or more nodes, I recommend radiation pretty highly,
and if they’re nodenegative I’m pretty much against
it. In my experience, younger women with greater numbers of positive
lymph nodes are more likely to opt for radiation.
Radiation therapy decisions are also often influenced by the type
of reconstruction that a woman has had. Women with implant reconstruction
are sometimes not quite as enthusiastic about radiation because
of the potential deleterious cosmetic effects.
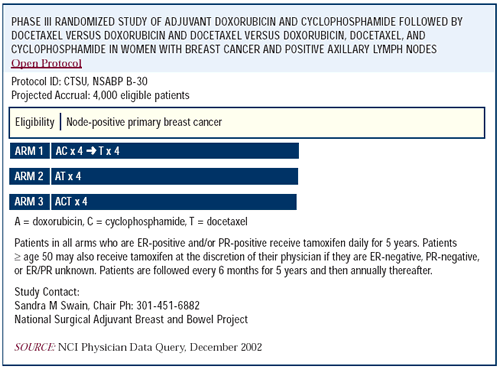
Nonprotocol use of AC-docetaxel
We participate in the NSABP B-30 trial, which involves AC followed
by docetaxel as its standard arm. In a nontrial setting, I would
frequently think about using the standard arm. For example, if I
was discussing NSABP B-30 with a patient, when we come back to a
discussion of standard therapy outside of the trial, we would talk
about the standard arm of this trial.
I would tell her about our uncertainty with regard to the taxanes.
Sometimes in a nonprotocol setting, we go in with the notion that
the patient is going to take the AC, we’ll see how it is going
and then she’ll come back and tell me how she feels about
taking the taxane. I have not been a big fan of six cycles of TAC
or FEC, but I know that many physicians are.
Docetaxel versus paclitaxel in the adjuvant setting
Some physicians are more interested in docetaxel than paclitaxel
for several reasons. One is the enthusiasm about the preoperative
docetaxel results from NSABP B-27. The second reason is that some
people have looked at the BCIRG trial of TAC versus FAC as an endorsement
of docetaxel.
I think that we’re doing an awful lot of early reporting.
The TAC results are interesting, but I want to see more follow-up.
I actually thought TAC would make a lot of headway in the community,
but — at least where I live — it doesn’t seem
to have made a big impact.
I'm most impressed that people are taking the subset analysis
from that trial very seriously. I’ve had people call me, reluctant
to use TAC in a patient with six positive lymph nodes, because in
that trial the advantage was only seen in the women with one to
three positive nodes. I'm impressed with how evidence-based many
of the physicians that I have spoken to are in making therapeutic
decisions.

Non-anthracycline containing regimens
We all feel reasonably confident that anthracycline-containing
regimens are probably slightly better than non-anthracycline-containing
regimens; however, I am still a fan of CMF in patients who have
cardiotoxicity issues. If you look at the differences — for
example a CAF versus CMF trial that we did through the Intergroup
— the absolute difference in benefit was actually very small.
Some patients may not find that worthwhile when considering the
tradeoff in terms of the cardiotoxicity concerns.
Effect of HER2 and nodal status on choice of
chemotherapy
I have not routinely used HER2 status to make chemotherapy decisions.
I do tell patients that there is some belief that HER2 positivity
might drive one to think harder about an anthracycline-containing
regimen. This finding, however, isn’t true across all studies,
and we know from our adjuvant trastuzumab trials that we’re
not very good at measuring HER2 status.
There has been as much as a 25-30 percent discordance rate between
local and central laboratory testing. This makes me very nervous
about putting a lot of emphasis on a study if I’m not completely
confident about the quality of the data.
If a patient had 15 positive nodes, I would probably think even
harder about any regimen that involves six months of therapy and
not so hard about four cycles of AC. I would also be thinking very
hard about her endocrine therapy. With regard to adjuvant trastuzumab,
I am a purist on this issue and a big believer in the randomized
trials — I have not given any adjuvant trastuzumab outside
the context of a clinical trial.
Ovarian suppression in ER-positive, premenopausal
women
Many younger women are still menstruating after the completion
of chemotherapy. Several years ago, I would only have discussed
tamoxifen, but presently I do actually discuss the uncertainty about
ovarian suppression strategies. I usually recommend tamoxifen, and
if a patient feels strongly, sometimes she’ll also undergo
ovarian suppression.
In higher-risk women, I would consider it more strongly. Based
on a very small retrospective subset analysis, women with 10 or
more positive lymph nodes were the ones who seemed to get a fair
amount of benefit from the combined endocrine therapy. The caveat
here is that even in our seemingly large trial that, that group
is only 100 women — a very small subset to base a lot on.
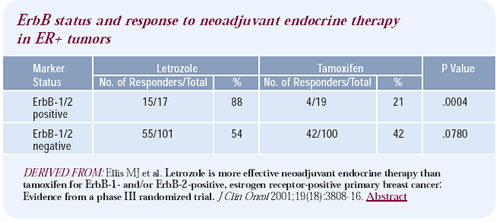
Impact of HER2 status on choice of endocrine
therapy
There is a belief that perhaps aromatase inhibitors are more effective
than tamoxifen in ER-positive, postmenopausal women whose tumors
overexpress HER2. This is based, in part, on Matt Ellis’ preoperative
study. There is really no equivalent data in premenopausal women.
Richard Love did a trial in Vietnam of premenopausal women where
the standard of care was no adjuvant therapy, and the experimental
arm was oophorectomy and tamoxifen. He found that there was no impact
of HER2 status on response to endocrine therapy. Combined endocrine
therapy was effective regardless of HER2 status. I don’t think
HER2 status should have any influence on the approach to adjuvant
endocrine therapy.
If a premenopausal woman stops menstruating after the completion
of chemotherapy, I would be oriented towards tamoxifen, but I would
have a discussion about tamoxifen versus anastrozole. Many of my
very sophisticated patients would want to talk about the impact
of HER2 status in that setting, and I’ve had a couple who
decided to go on anastrozole because of their belief that tamoxifen
may not be as good in that subset of ER-positive, HER2-positive
women.
Endocrine therapy in a woman with history of a
deep vein thrombosis
In a postmenopausal woman who has had a deep vein thrombosis in
the past several years, I would move to an aromatase inhibitor without
thinking very hard about it. If she had stopped menstruating after
chemotherapy, I would probably consider her postmenopausal.
It’s a little tougher in premenopausal women, and I would
think more about an ovarian suppression strategy as my only strategy.
Ovarian ablation or suppression as an alternative to tamoxifen in
premenopausal patients was endorsed by the 2000 NIH consensus conference.
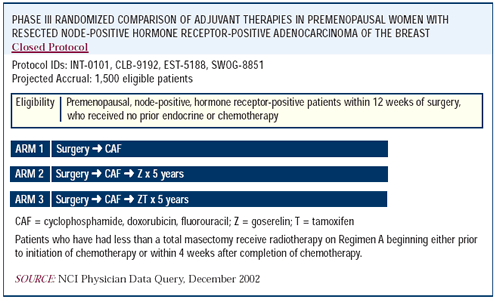
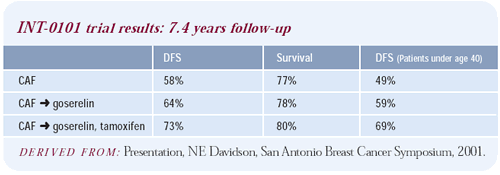
Intergroup Trial 0101
The design of this trial was CAF chemotherapy versus CAF chemotherapy
followed by five years of goserelin versus CAF chemotherapy followed
by five years of goserelin and tamoxifen. There is no impact on
disease-free survival in the overall population with the addition
of goserelin, but there is a trend to suggest that the younger patients
may benefit.
Although it seemed like such a large clinical trial at the time
it was initiated, 1,500 women doesn’t have the power to reveal
a significant difference even in those younger women and even with
all this follow-up.
We don’t have any new data over the last year on this question.
We have a lot of re-examination of old data. My synopsis is that
in ER-positive, premenopausal women, tamoxifen is a good drug. Ovarian
suppression or ablation is also beneficial, but we are having a
difficult time figuring out how to integrate them.
The one new trial that I’ve seen over the last year is the
Austrian trial published in the last couple of months comparing
CMF chemotherapy to ovarian suppression with tamoxifen in premenopausal
estrogen receptorpositive women. They suggested that the outcome
was slightly better with the combined endocrine therapy.
The difficulty with that trial is that the women who took chemotherapy
didn’t take tamoxifen because it was not the standard of care
when the trial was launched. Today we think of that as a pretty
profound deficit with that study and related studies. So we need
to come together to investigate this further. There is a trio of
trials that we are trying to launch worldwide to look at issues
of ovarian suppression in young women.
Combining LHRH agonists and aromatase inhibitors
in premenopausal women
I’m very enthusiastic about the research strategy of looking
at LHRH agonists with aromatase inhibitors. Extrapolating from the
early data in postmenopausal breast cancer, which suggested that
anastrozole may have superior efficacy compared to tamoxifen, this
seems like a rational strategy to transfer to premenopausal women
as well. The two issues are whether or not it is actually going
to be efficacious, and what is the cost in terms of side effects.
I wouldn’t utilize this strategy outside the context of a
clinical trial.
Assessment of menopausal status in ER-positive
patients and choice of endocrine therapy
In terms of determining whether a woman is pre- or postmenopausal,
I usually just assess patients clinically, not by testing with blood
work. If their menstrual periods go away, usually I’m already
giving tamoxifen if the patient is ER-positive, so I don’t
actually need to know her menopausal status to approach that. If
we were routinely using anastrozole in postmenopausal women —
and we are in that transition time right now — then we might
have to work a little harder to make sure they truly are postmenopausal.
The other issue is that women can become transiently postmenopausal
and have recovery of ovarian function at a later date.
I approach premenopausal women with metastatic disease who become
clinically menopausal after receiving chemotherapy as postmenopausal.
I have been using first-line aromatase inhibitors in these women
for several reasons. First, I’m impressed by the trial data
comparing them to tamoxifen as first-line therapy. Second, many
of those women have already been exposed to or are on tamoxifen
at the time of their relapse.
I start with either letrozole or anastrozole, and I can’t
tell you why sometimes I choose one over the other. I generally
do not use exemestane as my first choice. If the person has a good
response to their first aromatase inhibitor, I am hoping to capitalize
on the work from Per Lonning suggesting that women who have been
exposed to the nonsteroidal aromatase inhibitors can have a 20 percent
clinical benefit with exemestane.
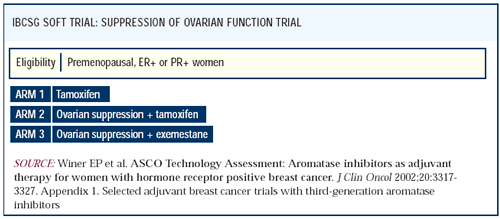
SOFT: Ovarian ablation with tamoxifen or an aromatase
inhibitor
The adjuvant ovarian suppression trial that I am most enthusiastic
about is SOFT — Suppression of Ovarian Function Trial. Premenopausal,
ER-positive women who may or may not have received chemotherapy
will be randomized to tamoxifen for five years, ovarian suppression/ablation
plus tamoxifen, or ovarian suppression/ablation plus an aromatase
inhibitor. This very interesting trial will help us address several
issues. Does ovarian ablation or suppression add to tamoxifen? And
if this is an important strategy, is it better to use tamoxifen
or an aromatase inhibitor in those suppressed women?
This trial is an international collaboration, put together by
the International Breast Cancer Study Group (IBCSG). The US cooperative
groups have signed on to it, and it is winding its way through the
approval process in the United States right now. I think it will
be launched within the next year.
Other trials of aromatase inhibitors with ovarian
suppression
There are two other studies of aromatase inhibitors with ovarian
suppression. One is built on the premise — which is pretty
popular in Europe — that since we know ovarian suppression
is important, some investigators would be unenthusiastic about a
trial that didn’t involve ovarian suppression. For those investigators,
the trial would be ovarian suppression with tamoxifen or ovarian
suppression with an aromatase inhibitor.
The other trial asks the question, “If you do ovarian suppression
with either tamoxifen or an aromatase inhibitor, do you really need
chemotherapy?” This trial randomizes to chemotherapy or not,
plus endocrine therapy.
I believe that will be a tough concept to sell in the United States,
but it may have some enthusiasts abroad. I personally think randomized
trials that involve two very different treatments, chemotherapy
or not, will be a little more difficult to conceptualize.
These trials were put together by the International Breast Cancer
Study Group. They have been looked at by the US cooperative groups,
and different groups will decide whether or not to endorse each
trial. Subgroups may decide that they are not as enthusiastic about
one design or another, and that they want to put all their effort
into one. My personal preference is the SOFT trial, because I think
it addresses the issues of interest to many US investigators.

Counseling postmenopausal women on adjuvant endocrine
therapy: Tamoxifen versus aromatase inhibitors
In counseling women about adjuvant endocrine therapy, it’s
a lot longer discussion now than in the past, because I feel obligated
to go through the ATAC trial in some detail and talk with people
about their preferences. Some women are pretty clear that they want
anastrozole, and I am comfortable prescribing it to them. Obviously,
if somebody has contraindications to tamoxifen, it’s a pretty
easy decision.
Many patients know a lot about tamoxifen. They know that it has
a long track record, and they’re pretty comfortable with that.
However, it's always stunning to me as an oncologist how much bad
press tamoxifen has received, for practically the least toxic drug
I can prescribe. It amazes me how many people will go through six
months of chemotherapy without batting an eyelash, yet come in very
concerned about the long-term consequences of tamoxifen.
The majority of my patients are going on tamoxifen right now,
but I think the sands are shifting. At the beginning, everybody
sort of sat tight, but since the FDA approval, I’ve seen more
women who are open to anastrozole by the time they come to see me.
When I do use an aromatase inhibitor in the adjuvant setting, I
only use anastrozole. I’m a purist on that. The one trial
we have adjuvant data from utilized anastrozole, so I want to do
it exactly as we did in that trial.
Use of bisphosophonates in the adjuvant setting
There is a trial in Austria randomizing premenopausal, ER-positive
patients to various endocrine therapies with a second randomization
to different schedules of zoledronate. Their long-term goal is not
only impact on bone density, but also on breast cancer recurrence,
based on all these conflicting results with clodronate.
In my practice, I usually watch bone mineral densities, and if
they get down to a range I’m unhappy with, I use one of the
oral bisphosphonates. I still use a lot of adjuvant tamoxifen, and
for premenopausal women that is a pretty good bone drug. They may
not need anything in addition until they come off of tamoxifen.
I haven’t used a lot of adjuvant aromatase inhibitors, but
I think this is where it might turn out to be an issue in the future.
Approach to chemotherapy in younger versus older
women with metastatic disease
My philosophy in treating older versus younger women with chemotherapy
is basically the same, but sometimes the patient choices are different.
Many times in metastatic disease, we use all of the available therapies,
so what we’re really deciding on is the order — what
to start with. Many patients make that decision based on their personal
values. I find many of my older patients are attracted to capecitabine
because it is an oral agent. Some of my younger patients think of
intravenous therapy as more aggressive, and they prefer that strategy.
But, this perception is people’s gut reaction rather than
being reality-based.
Capecitabine in the metastatic setting
I am a big fan of capecitabine. Maybe it comes from being a "hormonal-therapy
person" preferring pills to begin with, because I use it a
lot for salvage chemotherapy in women who’ve already had an
anthracycline and taxane for metastatic disease. In oncology, we
tend to remember our successes, but I have seen several very impressive
responses with capecitabine in pretty dire circumstances. I have
had women on it for a considerable period of time with relatively
good quality of life. My personal best was somebody who was on capecitabine
for several years.
Combination versus sequential therapy in the
metastatic setting
ECOG-1193 trial compared doxorubicin followed by paclitaxel at
disease progression versus paclitaxel followed by doxorubicin at
disease progression versus the combination. There is no question
that the response rate was higher with the combination, but at the
end of the day, survival was identical in the three arms. This says
to me that how you package those drugs is probably not as important
as long as people are exposed to both of them in the metastatic
setting.
I am philosophically more inclined toward sequential single-agent
therapy in metastatic breast cancer. However, I'm fascinated by
the capecitabine/ docetaxel trial. Most of the women on that trial
who took docetaxel alone did not get exposure to capecitabine, and
I suspect that if there had been a crossover arm, the survival would
not have been much different. Having said that, I am an enthusiast
about the adjuvant and neoadjuvant trials looking at the combination
of capecitabine/docetaxel.
Adjuvant capecitabine trial in elderly women
I would probably be willing to put women of any age on this trial,
but I think the trial focuses on elderly patients for two reasons.
One is that the elderly are a research focus of Hyman Muss, the
principal investigator of the trial. The other is that he thought
oral therapy, which is a little less intrusive, might be more in
keeping with the lifestyle issues faced by the elderly patient.

We haven’t had a lot of single-agent adjuvant therapy for
quite some time, so that always gives people pause. We are revisiting
whether some of our newer single agents — when given optimally
— might be every bit as good as some of our combination therapies.
In the Intergroup, we are about to launch a trial in node-negative
patients that is a two-by-two design involving either four or six
cycles of AC versus 12 or 18 weeks of paclitaxel. The question is
whether or not you can preserve the benefits of adjuvant chemotherapy
with a better toxicity profile, particularly the concern about longer-term
cardiotoxicity.
People were impressed by the preclinical and early clinical information,
which suggested that weekly taxanes may be better than an every
three-week schedule. I am interested to see whether 18 weeks of
paclitaxel is the kind of therapy that you can just breeze through.
It may not be quite as simple as we think.
Select publications
|
