|
You are here: Home: BCU 7|2002: Debu Tripathy, MD
 |
 |
 |
 |
 |
Debu
Tripathy, MD |
 |
 |
Professor of Medicine and Director,
Komen Alliance Breast Cancer Research Center,
University of Texas Southwestern Medical Center |
|
 |
 |
 |
Edited comments by Dr Tripathy
| CASE 1: 45-year-old premenopausal
woman with recurrent breast cancer who participated in the trastuzumab
pivotal trial |
History
At initial presentation in 1995, this woman had a 2-centimeter,
high-grade, ER/PR-negative, HER2-positive (IHC 3+) tumor and
three positive lymph nodes (stage IIB). She was treated with
a mastectomy and adjuvant doxorubicin/cyclophosphamide.
Approximately one year after completing adjuvant chemotherapy,
she had a chest wall recurrence and pulmonary metastases.
She had neglected to come in for follow-up, and the recurrence
was fairly extensive over the entire left chest wall, involving
the side and extending to the scapula. There were some areas
of ulceration anteriorly. She was having a fair amount of
discomfort over her chest wall, but she was not having any
symptoms from her pulmonary disease.
Follow-up
She enrolled on the randomized trial comparing chemotherapy
alone to chemotherapy plus trastuzumab. She was randomized
to paclitaxel alone and progressed. Then, she was eligible
to cross over to paclitaxel/trastuzumab. However, she did
not respond to that combination either.
The pulmonary nodules doubled in size to around four centimeters.
Her chest wall disease also expanded to involve the lower
aspect of the chest wall and more of the scapular area. She
also developed lymphadenopathy in the axillary and supraclavicular
areas on the left side.
The protocol allowed the chemotherapy regimen to be changed,
and then she was treated with vinorelbine/trastuzumab. She
had a dramatic response to that regimen and an excellent quality
of life. After about two and a half years, she again progressed
in the chest wall, lung and also the liver. She ultimately
died of hepatic failure about two years ago.
Case discussion
By the time this patient presented with local recurrence,
local radiotherapy was not an option. When she was staged,
she also had pulmonary metastases. She was clearly a candidate
for some form of systemic therapy, and she was eligible for
the randomized trial with trastuzumab. Since she had already
received doxorubicin, she was randomized between paclitaxel
alone and combination paclitaxel/trastuzumab.
We were, of course, discouraged that she did not respond
to either paclitaxel alone or — on crossover —
to paclitaxel/trastuzumab. Given some of the uncertainty and
the lack of options, it was reasonable to try a combination
of vinorelbine/trastuzumab. At that time, here was very early
data about the synergy with these two agents from Mark Pegram’s
laboratory.
Part of the reason this case is so memorable is that she
had a very dramatic response to vinorelbine/trastuzumab. Her
chest wall disease essentially disappeared completely. I have
never really seen such a response in someone whose disease
was so extensive. She had some residual pinkness to the skin,
but really no nodular changes. Her pulmonary nodules did not
go away completely, but they regressed by 80% or 90%. She
had a major — almost complete — response. |
Choice of adjuvant chemotherapy
Currently, I am using four cycles of doxorubicin/cyclophosphamide
mostly for patients with negative lymph nodes. If a patient with
ER-negative, node-positive disease presented today, I would use
adjuvant doxorubicin/ cyclophosphamide, followed by paclitaxel.
In patients with ER-positive disease, I think it is reasonable
to use paclitaxel. I am, however, concerned about the subanalysis
showing a lack of benefit in patients with ER-positive disease.
Although the statisticians tell us not to look at that subset, CALGB
9344 had 2,000 ER-positive and 1,000 ER-negative cases. Early data
from NSABP B-28 seems to show a trend in the same direction.
Even though I think taxanes are reasonable, and, in fact, they
are the control arm of many of the Intergroup studies, for my patients
with node-positive, ER-positive disease, I also think it is reasonable
to use six cycles of an anthracycline-containing regimen, like FAC
or FEC. That is typically what I am using.
Choice of adjuvant hormonal therapy in premenopausal
patients
In patients with ER-positive disease and positive lymph nodes,
I would use tamoxifen following the adjuvant chemotherapy. In women
who are still menstruating, I think the LHRH agonists are reasonable
as well, but the value of adding an LHRH agonist to tamoxifen is
still questioned. In the large European study, the Zoladex®
in Premenopausal Patients (ZIPP) trial, there was a clear advantage
with goserelin, but in the subgroup of women on tamoxifen, it was
not statistically significant.
A proposed Intergroup study will evaluate chemotherapy followed
by tamoxifen, with or without an LHRH agonist, in women who are
still menstruating after chemotherapy. Now that the ATAC trial results
are available, they are also thinking about evaluating an LHRH agonist
plus anastrozole in those women. I would support a trial where the
control arm would be tamoxifen and the experimental arms would be
an LHRH agonist plus tamoxifen, and an LHRH agonist plus an aromatase
inhibitor.
Postmastectomy radiation therapy
Unless a patient has a primary tumor that is more than five centimeters,
or with skin involvement or more than four positive lymph nodes,
I do not routinely use radiation therapy postmastectomy. It is still
unclear whether a positive HER2 status alone would be enough of
a risk factor for local recurrence to warrant the use of radiation
therapy.
The use of postmastectomy radiation therapy in patients with one
to three positive lymph nodes is an area that is under investigation.
Patients with one to three positive lymph nodes, and a primary tumor
that is less than five centimeters, are the subjects of a randomized
trial comparing postmastectomy radiation therapy to observation.

Responses to second-, third- and fourth-line chemotherapy
I have seen maybe two or three patients with a dramatic response
to second-, third- or fourth-line chemotherapy. For example, we
start them on capecitabine, and they have a great response —
independent of trastuzumab.
I have seen HER2-negative patients progress on doxorubicin and
then on paclitaxel, and then have a great response to capecitabine.
Even though these patients are not common, we do occasionally encounter
patients who respond to their third- or fourth-line chemotherapy
much more dramatically than prior agents. We need to develop markers
of resistance and sensitivity to therapy, so we can know ahead of
time what drugs to use.
Choice of systemic therapy for patients with
HER2-positive metastatic disease
In patients with HER2-positive metastatic disease, it is a given
that those patients will receive trastuzumab. Whether to add chemotherapy
is the question. In the absence of cardiac disease, I would use
trastuzumab monotherapy in a patient who is asymptomatic or whose
disease was not immediately life-threatening.
An asymptomatic patient with bone-only or soft-tissue disease
would be — in my mind — an ideal candidate for trastuzumab
monotherapy. In a patient with multiple liver lesions who might
get into trouble with a little progression — even though they
were asymptomatic — I might consider combination therapy with
chemotherapy plus trastuzumab.
For combination therapy, I use a taxane with trastuzumab. Since
the data is based on paclitaxel, I tend to use that. There are early
trial results from combinations with docetaxel showing response
rates from 35% to 60%. Certainly, I think docetaxel is active in
combination with trastuzumab.
The group at UCLA strongly believes that the synergy with docetaxel
— at least in the laboratory — is greater than with
paclitaxel. Therefore, they have designed their trials, both in
advanced and early-stage disease, with docetaxel.
The high response rates with the combination of vinorelbine/trastuzumab
are encouraging. Soon, we may have reason to use it as first-line
therapy. There are proposed trials comparing a taxane to vinorelbine,
in combination with trastuzumab, as front-line therapy. Vinorelbine/trastuzumab
and paclitaxel/trastuzumab are both very tolerable. Bone marrow
toxicity may occur a little earlier with vinorelbine.
Trastuzumab pivotal trial
The primary outcome initially chosen was time to disease progression.
Survival, even though it was captured, was not expected to be different.
In fact, that is why the crossover was allowed. When the survival
difference emerged, despite the fact that close to 70% of the patients
crossed over, it was indeed remarkable.
In retrospect, it would have been interesting to have trastuzumab
monotherapy as the third arm of that trial. In fact, such a trial
is now being proposed. For example, trastuzumab alone will be compared
to trastuzumab plus a taxane. Then, upon progression on trastuzumab
alone, there will be a second randomization to either a taxane alone,
or a taxane plus trastuzumab. I think that that will be an excellent
trial.
Trastuzumab in combination with other agents
Another area of interest is the combination of trastuzumab with
other drugs. Some of these combinations are driven by in vitro data.
The combinations with gemcitabine and the platinums look interesting.
Much of the original preclinical work was done with cisplatin.
In fact, an early Phase II study by Mark Pegram demonstrated activity
for trastuzumab in combination with cisplatin in very refractory
patients. The BCIRG and the group at UCLA are capitalizing on this
in their adjuvant trial.
There is also data from a trial in metastatic disease looking
at trastuzumab in combination with either carboplatin, or cisplatin
along with docetaxel, which found very high response rates.
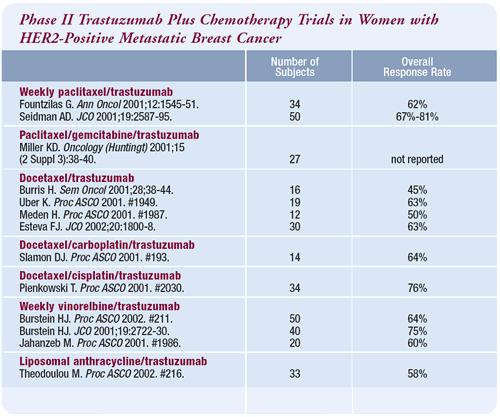
Trastuzumab/vinorelbine
Probably the most exciting data is the high degree of activity
demonstrated with the trastuzumab/vinorelbine combination. This
has been confirmed in a separate trial, but I think it still needs
to be confirmed in the large ongoing multicenter trial. Furthermore,
I believe there is a trial comparing paclitaxel or docetaxel to
vinorelbine in combination with trastuzumab.
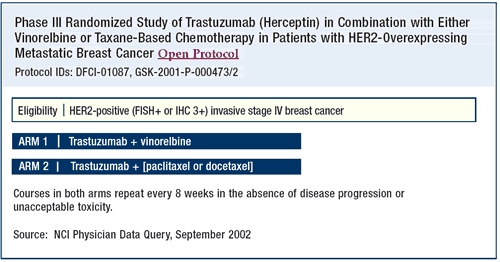
Neoadjuvant trastuzumab
The neoadjuvant data for trastazumab exemplify a totally different
set of circumstances. With chemotherapy alone, clinical response
rates are in the 70% to 90% range. It is not surprising then that
the trastuzumab combinations show those same response rates. Since
the pathologic complete response rate is a surrogate for survival,
we are very interested in that.
I support these trials. I do not treat patients outside of a trial
with neoadjuvant trastuzumab, but we are certainly going to participate
in the CALGB trial with neoadjuvant trastuzumab. That trial is designed
to determine the efficacy of dexrazoxane (Zinecard®), trastuzumab
in combination with paclitaxel, and one year of trastuzumab following
surgery.
First, the patients are randomized to receive doxorubicin/cyclophosphamide,
with or without dexrazoxane. This part of the trial will determine
whether the introduction of a cardioprotectant can have a long-term
effect on controlling cardiotoxicity. In the second phase of the
study, the patients will receive paclitaxel/trastuzumab. Then, the
patients will have surgery, and they will receive trastuzumab for
one year.
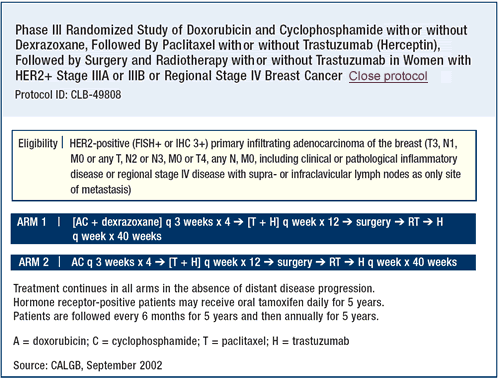
Algorithm for HER2 testing
We assume that the tumors with a 3+ score on immunohistochemistry
(IHC) are truly HER2-positive, and we do not test them further.
If a tumor has a 2+ score on IHC, we test with fluorescence in situ
hybridization (FISH). Even in patients with an IHC score of 0 or
1+ and other features of excessively aggressive disease, we may
also do a FISH test.
An IHC score of 3+ is pretty reliable, as long as it is done at
a laboratory that performs a lot of assays. Both the Intergroup
and the NSABP study discovered that smaller community hospitals
were overscoring tumors as 3+. Close to 30% of the 3+ scores were
downstaged when they were reviewed centrally. These protocols have
now been amended to require that the patients wait for final randomization
until there is a central review of their HER2 status.
I think the same things apply to FISH testing. Since FISH testing
already tends to be done at more centralized laboratories, we have
not yet explored the quality control issues. I suspect there will
be a proliferation of FISH testing, and the reagents will go out
to all the community hospitals. Even though there is probably less
room for interobserver variability, the same issues will apply.
I hope as the FISH technology disseminates, people will do these
quality control-type studies.
At some point, it may be possible that the only test that will
be done is FISH. Personally, I believe it to be more accurate and
less subject to interobserver variability. I think the cost should
be downplayed if it is only a difference of $100 or $200. However,
when trastuzumab is given incorrectly for several months, that involves
many thousands of dollars. It behooves us all — even from
a cost standpoint — to get the most accurate test up and running.
Schedules of trastuzumab plus weekly taxanes
It was expected that weekly taxanes would be effective with trastuzumab,
and, in fact, it was found to be the case. This paves the way for
using weekly taxane regimens in the adjuvant setting and also for
metastatic disease.
Trastuzumab administered every three weeks
Trastuzumab administered at longer intervals (every three weeks)
and at three times the dose is being investigated. Brian Leyland-Jones
presented data on paclitaxel with trastuzumab given every three
weeks that demonstrated the trough did not go below the desirable
level. In fact, the overall area under the curve and the peak concentration
are higher without any additional toxicity. This may allow for the
convenience of every-three-week administration.
I still, however, use weekly trastuzumab. I want a little more
toxicity data. For many drugs, it is the peak level that actually
mediates toxicity. That may not be the case with trastuzumab, but
I would like a little longer follow-up, especially for cardiotoxicity.
Adjuvant trastuzumab
Whether it makes sense to use adjuvant trastuzumab in a woman
whose odds of dying from breast cancer are less than the odds of
dying from atherosclerotic heart disease is a big question. We do
not know the answer yet. Therefore, adjuvant trastuzumab is being
evaluated in high-risk women with node-positive disease, where the
potential benefits might be at least proportionally larger.
It may be reasonable to use adjuvant trastuzumab off-protocol
for a young woman with multiple (i.e., 15) positive nodes and HER2-positive
disease, or for a young woman with HER2-positive, inflammatory breast
cancer. Although I personally have not done that, I think it is
sound medical judgment as long as the patient is informed of the
potential toxicities.
Clinical implications of the ATAC trial results
The biggest change in breast cancer has been the advances in hormonal
therapy. I was surprised, when the early results from the ATAC trial
were reported, that the benefits with anastrozole were evident so
early.
I think the data from the ATAC trial is very convincing. It is
a huge trial with more than 9,000 patients, and it is very unlikely
that the curves will change over time. However, I am not sure what
the long-term toxicities will be. The data already suggests that
there may be a higher risk of fracture in women on aromatase inhibitors.
Currently, my approach is to use tamoxifen for low-risk patients
with nodenegative disease. In higher-risk patients (i.e., multiple
positive nodes), I could probably make a case that, even with a
small risk of osteoporosis, there is more to gain from an aromatase
inhibitor. The benefits are proportional to the risks.
The final issue is the risk of tamoxifen. Tamoxifen is generally
a safe drug, but in women over the age of 70, there is an excess
risk of stroke. I think in women over the age of 70, I am also compelled
to consider an aromatase inhibitor. Even in lower-risk women, my
threshold for risk goes a little lower in those over the age of
70, mostly because of the risk of stroke.
In premenopausal women with multiple positive nodes, I would consider
medical oophorectomy. In those types of patients, it might be reasonable
to use an aromatase inhibitor. In premenopausal women with multiple
positive nodes who stop menstruating after chemotherapy and have
low estradiol levels, I would also consider an aromatase inhibitor.
Interchangeability of the aromatase inhibitors
In the adjuvant setting, I am currently using anastrozole, but
I think the aromatase inhibitors are generally equivalent. At least,
we have data on anastrozole. Soon, we will have data with some of
the other aromatase inhibitors in the adjuvant setting.
Fulvestrant: Sequencing of hormones therapy in
metastatic disease
Fulvestrant (Faslodex®) creates a dilemma, in that the pivotal
trials were conducted in tamoxifen-refractory patients. Fulvestrant
will be used in patients after an aromatase inhibitor, and there
is no data on the efficacy of fulvestrant given after an aromatase
inhibitor. How effective fulvestrant will be in women who have progressed
on an aromatase inhibitor is the key question that needs to be answered.
Biologically speaking, fulvestrant removes the estrogen receptor.
It is an estrogen-receptor downregulator. Once fulvestrant complexes
with the estrogen receptor, the receptor is actually degraded. In
contrast, the estrogen receptor and tamoxifen complex is translocated
to the nucleus. The aromatase inhibitors basically remove estrogen,
and fulvestrant removes the estrogen receptor. Therefore, nothing
goes to the nucleus with either an aromatase inhibitor or fulvestrant.
In a woman who has relapsed on adjuvant tamoxifen and has never
received an aromatase inhibitor, I would generally use an aromatase
inhibitor. In this type of situation, fulvestrant was found to be
roughly equivalent to an aromatase inhibitor, and the American trial
suggested that the time to disease progression might actually be
a little bit longer for fulvestrant. Since that was not the primary
end point, I think we have to look at that information cautiously.
Fulvestrant and the aromatase inhibitors, in my mind, really represent
equivalent therapeutic choices.
Select publications
|
