|
You are here:
Home: BCU 7|2002: Rowan T Chlebowski, MD, PhD
Edited comments by Dr Chlebowski
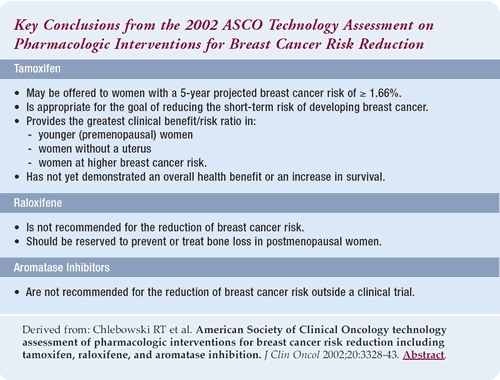
2002 ASCO technology assessment of pharmacologic
interventions for breast cancer risk reduction including tamoxifen,
raloxifene and aromatase inhibition
Evolution of chemoprevention research
In 1999, there were about 350 events in the NSABP-P1 trial which
demonstrated a 50% risk reduction with tamoxifen. At the same time,
there were less than 100 events on the European trials which showed
almost no effect. Now, results from the International Breast Cancer
Intervention Study (IBIS-1) have been published. Additionally, Jack
Cuzick’s meta-analysis has updated the other two European
trials.
In the European studies, risk assessment was completely based
on family history. Therefore, they had a different patient population.
In the NSABP-P1 and IBIS-1 trials, 17% and 5% of the women, respectively,
had LCIS or atypical ductal hyperplasia. In the Royal Marsden trial
and the Italian trial, none of the women had those conditions.
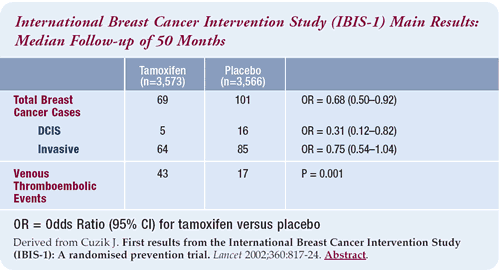
IBIS-1 randomized over 7,000 women with an increased breast cancer
risk to tamoxifen or placebo. Although 40% of the women were receiving
hormone replacement therapy, there still was a 33% statistically
significant reduction in breast cancer.
When all of the trials are pooled together, there is an overall
38% reduction in breast cancer risk. There is a statistically significant
50% reduction in the risk of ER/PR receptor-positive breast cancer
and a statistically nonsignificant 25% increase in ER/PR receptor-negative
breast cancer with tamoxifen. Interestingly, all-cause mortality
is the same for women receiving tamoxifen or placebo. However, there
may not yet be enough deaths to make a statement about tamoxifen’s
effect on overall mortality.
The statistically nonsignificant increase in ER/PR receptor-negative
breast cancers is an interesting issue. Last year, in the Journal
of the National Cancer Institute, the Seattle group reported on
an epidemiological study suggesting the same thing. One potential
explanation is that suppressing the receptorpositive breast cancers
leads to a differential increase in ER/PR receptornegative cancers.
Clinical implications of chemoprevention trials
The 2002 ASCO technology assessment states that tamoxifen be recommended
for the short-term (five-year) reduction of breast cancer risk.
The indications for tamoxifen have narrowed, because we are more
cognizant of its side effects and the fact that younger women and
women without a uterus derive the most benefit.
The patient-care message from the 2002 ASCO technology assessment
is that tamoxifen effectively reduces the risk of breast cancer,
but women must consider the risks and benefits. I think younger
women (under the age of 50) with an increased risk of breast cancer
based on family history or several biopsies, will benefit the most.
Older women, without a uterus and with a strong family history,
may benefit as well. At this point, tamoxifen is for short-term
breast cancer risk reduction as opposed to general health benefit.
IBIS-2 chemoprevention trial
The IBIS-2 trial plans to identify a high-risk population based
on family history and to randomize them to anastrozole or placebo.
There is a more complicated schema for patients with DCIS, in which
tamoxifen may be the control arm.
The Study of Tamoxifen and Raloxifene (STAR) trial is ongoing
and has recruited over 13,000 of it’s target 22,000 patients.
The STAR trial does not have a placebo control. However, IBIS-2
is going ahead with a placebo control. The 58% percent reduction
in contralateral breast cancer reported for anastrozole in the ATAC
trial suggests that anastrozole may be better than tamoxifen, with
a different toxicity profile. Since the effects of tamoxifen on
overall health are unknown, it may be appropriate to go forward
with the placebo control.
Tamoxifen and the risk of uterine sarcoma
Tamoxifen has an — undeserved — bad reputation. The
recent FDA warning about the risk of uterine sarcoma will make that
reputation worse. I do not think it changes the risk-benefit ratio,
but, it might make women hesitant about taking tamoxifen.
In tracking toxicity reports, the FDA saw a number of uterine
sarcomas — a relatively uncommon malignancy. Then, they discovered
that, of the tamoxifen-associated endometrial malignancies, approximately
20% were uterine sarcomas. Since this represented a worse-prognosis
cancer, they wanted to focus attention on the DCIS and the risk-reduction
indication.
Hormonal therapy and cognition
Evidence from preclinical, clinical observational and randomized
trials suggests that estrogen may play a role in maintaining cognition
in postmenopausal women. There are large-scale randomized trials
involving thousands of women that will address this question, but,
we do not currently have prospective data.
One of the issues for tamoxifen, since it increases hot flashes
by about 20%, is that it crosses the blood-brain barrier and interacts
with the central nervous system (CNS). Therefore, tamoxifen could
act as an estrogen antagonist in the CNS and be associated with
poor cognition. However, that has not been found with the screening
questionnaires done in NSABP-P1 and other trials, but, those questionnaires
are generally insensitive and not designed to answer that specific
question.
Effects of tamoxifen on brain metabolism
To test the hypothesis that tamoxifen might impair cognition,
we utilized a very interesting neuroimaging technique — proton
magnetic resonance spectroscopy — that can measure the concentrations
of biochemical markers associated with brain injury.
We measured myo-inositol levels, which increase in response to
brain injury. An increase in myo-inositol levels is predictive of
progression in AIDS dementia and Alzheimer’s disease and is
linearly related with age.
We studied 75 women (= 65 years of age) who received hormone replacement
therapy for two or more years, tamoxifen for two to five years or
no hormonal therapy. We found a time-dependent, statistically significant
reduction in myo-inositol levels in women treated with tamoxifen.
There was a similar trend in the women treated with estrogen.
These results would lead us to predict that tamoxifen will have
a neuroprotective effect in the CNS. Perhaps, tamoxifen is agonistic
on this part of the brain, but antagonistic on the part that controls
vasomotor symptoms.
The role of lifestyle modifications in
breast cancer prevention
Reduction in dietary fat intake
There are over 50,000 women currently randomized to studies evaluating
the role of dietary fat intake reduction in primary or secondary
breast cancer risk reduction. The Women’s Health Initiative
(WHI) randomized 47,000 healthy, postmenopausal women to dietary
fat intake reduction or observation. A report on all-cause mortality
will be available in 2005.
The Women’s Intervention Nutrition Study (WINS) has accrued
close to 2,400 postmenopausal women who received standard adjuvant
chemotherapy. After the women complete their standard interventional
therapy, they are randomized to dietary fat intake reduction or
observation. We have over 200 events and are due to report in a
year or two. The Women’s Healthy Eating and Living (WHEL)
study has a similar design, and randomizes postmenopausal women
up to three years after breast cancer diagnosis.
I think these studies have a good chance of being positive. One
of the reasons I am enthusiastic is the attention focused on molecular
medicine and biology and the cross-talk between all the receptor
pathways. It is easy to see how the insulin-regulatory pathway is
going to play a role in this process.
Pam Goodwin reported in the Journal of Clinical Oncology a prospective
observational study. She found that women with fasting insulin levels
in the highest quintile, compared to the lowest, had an eight-fold
increased risk of breast cancer recurrence and death. Therefore,
insulin itself might be a mitogen. In the nonprotocol setting, I
will chat with breast cancer survivors about modifying their dietary
fat intake.
Exercise
Observational studies demonstrate that increasing exercise may
decrease the chance of breast cancer recurrence. Therefore, there
are ongoing pilot studies. There are many potential mechanisms for
exercise’s antitumor effects, including a change in estrogen
levels, insulin-regulatory pathways and COX-2 inhibition.
In noncancer patients, exercise is a great mediator of hot flashes,
but, this has not been discussed in the breast cancer setting. There
was a report at ASCO 2002 demonstrating that exercise increased
bone mineral density in women receiving chemotherapy for breast
cancer. There was also another report indicating that a physician’s
recommendation to exercise resulted in a change in women’s
intentions to exercise.
Select publications
|
