|
You are here: Home: BCU 7|2002: Clifford A Hudis, MD
Edited comments by Dr Hudis
2002 ASCO technology assessment on the use of
aromatase inhibitors as adjuvant therapy for women with hormone
receptorpositive breast cancer
Winer EP et al. American Society of Clinical Oncology
technology assessment on the use of aromatase inhibitors as adjuvant
therapy for women with hormone receptor-positive breast cancer:
Status report 2002. J Clin Oncol 2002;20:3317-27.
In developing these guidelines, our group struggled with issues
related to the role the aromatase inhibitors would play. Eric Winer
highlighted that in the short run, maybe the most important outcome
of the ATAC trial, is that now we have a proven alternative.
In the past, we had some data for toremifene versus tamoxifen,
but the ATAC trial shows us that there’s a drug that could
be better and that certainly appears in many ways to be safer —
and specifically safer in terms of toxicities that bother patients.
I have a very informed patient population. My patients come in
asking questions, and I like those patients in terms of my own style
of practice. It’s the one that works for me.
So, I share this information with them, not only for the purpose
of education, but also because even if I don’t talk about
it with them and even if they don’t bring it up, it’s
very likely that after they leave my office in the weeks or months
that follow, they’re going to hear about this. I’d rather
talk about it with them at the beginning.
| Key
issues addressed by the 2002 ASCO technology assessment on the
adjuvant use of aromatase inhibitors |
Conclusions
“The panel was influenced by the compelling,
extensive, and long-term data available on tamoxifen. Overall,
the panel considers the results of the ATAC trial and the
extensive supporting data to be very promising but insufficient
to change the standard practice at this time (May 2002). A
five-year course of adjuvant tamoxifen remains the standard
therapy for women with hormone receptor positive breast cancer.
The panel recommends that physicians discuss the available
information with patients, and, in making a decision, acknowledge
that treatment approaches can change over time. Individual
health care providers and their patients will need to come
to their own conclusions, with careful consideration of all
of the available data.”
Women who have already started taking
adjuvant tamoxifen
In a woman who has already started a course of adjuvant
tamoxifen, no data currently supports the substitution of
an aromatase inhibitor (A.I.) for tamoxifen. Women experiencing
intolerable side effects related to tamoxifen may consider
switching to an A.I..
Women who have completed a 5-year course of adjuvant tamoxifen
and are disease-free In a woman who has completed a 5-year
course of adjuvant tamoxifen and is disease-free, an A.I.
should not be considered unless such therapy is part of a
clinical trial.
Premenopausal women
In premenopausal women with functioning ovaries, the A.I.s
are contraindicated. In the adjuvant setting, an A.I. in combination
with either an LHRH agonist or oophorectomy should not be
considered outside the context of a clinical trial.
In women who are premenopausal at diagnosis and experience
a disruption in ovarian function from chemotherapy, adjuvant
A.I.s should also not be used. There is concern about the
use of A.I.s in women with a probability of resuming ovarian
function.
Women with HER2-positive breast cancer
HER2 status should not be used in making decisions about
adjuvant hormonal therapy. Women with a relative or absolute
contraindication to the initiation of adjuvant tamoxifen In
postmenopausal women with a contraindication to adjuvant tamoxifen,
an adjuvant A.I. may be reasonable. Careful consideration
should be given to the significance of any relative contraindication
compared to the proven benefits of tamoxifen.
Women who develop ER/PR–positive
invasive breast cancer while taking either tamoxifen or raloxifene
Postmenopausal women developing ER/PR–positive cancers
while taking tamoxifen or raloxifene are considered clinically
resistant to these antiestrogenic agents. Therefore, it is
reasonable to use an adjuvant A.I..
Duration of therapy with an A.I. in
the adjuvant setting
If an A.I. is used as adjuvant therapy, it should be administered
for 2 to 3 years based on the ATAC trial results. The issue
of duration of therapy should be reassessed when more data
becomes available.
Anastrozole versus other A.I.s
In the adjuvant setting, the only available data for the
third-generation A.I.s are with anastrozole. Although the
A.I.s are closely related, they may have different toxicity
profiles. Therefore, anastrozole is the preferred A.I. in
the adjuvant setting. |
| DERIVED
FROM: Winer EP et al. American
Society of Clinical Oncology technology assessment on the use
of aromatase inhibitors as adjuvant therapy for women with hormone
receptor-positive breast cancer: Status report 2002.
J Clin Oncol 2002;20:3317-27. |
Adjuvant hormonal therapy in women previously
taking tamoxifen or raloxifene
This comes at exactly the right time, because we may now have
patients who have already taken tamoxifen and/or raloxifene, either
for prevention of breast cancer or osteoporosis. A burning question
has been: What to do with the breast cancers that develop in those
patients, if they are ER-positive? Now we know that those patients
can be offered an aromatase inhibitor as adjuvant therapy.
Another issue is the woman who develops a second primary ER-positive
breast cancer while on tamoxifen, many physicians would have immediately
used an aromatase inhibitor in the past. ATAC provides a little
more solid evidence to support that decision.
Adjuvant hormonal therapy in women with contraindications
to or intolerable toxicities from tamoxifen
In a patient who has a history of a hypercoagulable state or unacceptable
toxicities with tamoxifen, one might consider switching to an aromatase
inhibitor. Although, I am not convinced that switching to an aromatase
inhibitor will alleviate patients’ hot flashes.
In women complaining of weight gain with adjuvant tamoxifen, the
first thing we do is counsel them. There is no guarantee that some
of those complaints will not continue when the patient switches
to an aromatase inhibitor. If, after a long discussion, the patient
says they are going to stop taking tamoxifen, I certainly would
encourage an aromatase inhibitor.
Adjuvant hormonal therapy in women with HER2-positive
breast cancer
I agree with the findings of the ASCO technology assessment guidelines
that at present time, HER2 status should not be used to determine
adjuvant therapy in early stage breast cancer.
Adjuvant therapy in an elderly patient with a
2-cm tumor (ERpositive, HER2-positive) and two positive lymph nodes
I would treat this type of patient with adjuvant combination chemotherapy
and five years of tamoxifen. The age cut-off at which I would not
recommend adjuvant chemotherapy depends on what the patient looks
like. That is obviously not true in the extreme; I cannot imagine
a situation in which I would give adjuvant chemotherapy to a 98-year-old
woman.
This decision more involves the physiologic age than the chronoloic
age. It also requires a very informed discussion with the patient
about the risks and benefits of therapy. I can imagine treating
patients into their eighties with adjuvant chemotherapy, but I would
certainly be less likely to do so as they get older.
Outside of a clinical trial, I would use adjuvant doxorubicin/cyclophospha-mide
followed by paclitaxel (ACT), backing off only if there was a specific
toxicity in a particular patient. I am not convinced that ACT is
much more toxic than AC. Elderly patients would also be eligible
for Hy Muss’ trial, comparing single-agent capecitabine to
either CMF or AC.
Adjuvant systemic therapy of patients with HER2-positive
breast cancer
In patients who also have HER2-positive breast cancer, unless
there is a specific contraindication, I would be biased in favor
of using an anthracycline. Although many experts believe that HER2
status is not a proven predictive factor for response to chemotherapy,
I think there is more than a little evidence to suggest that patients
with HER2-positive disease may derive marginal benefit from anthracyclines.
TAC compared to FAC
As demonstrated in Jean Marc Nabholtz’s abstract comparing
TAC (docetaxel/doxorubicin/cyclophosphamide) to FAC (5-fluorouracil/
doxorubicin/ cyclophosphamide). Overall, there was a statistically
significant improvement in disease-free survival associated with
TAC.
Interestingly, it was most striking in the group with one to three
positive lymph nodes, and a preplanned subset analysis showed no
benefit in the group with four or more positive lymph nodes. The
TAC versus FAC trial is another brick in the foundation indicating
that there is a small and clinically important advantage to the
taxanes. Patients with HER2-positive disease seem to get a marginal
benefit from taxanes also.
First-line therapy for recurrent ER-positive,
HER2-positive breast cancer
In a patient relapsing two years after adjuvant ACT and while
on tamoxifen, with bone-only disease that is easily managed, I would
certainly be inclined to try an aromatase inhibitor as my first
maneuver. I would use either anastrozole or letrozole.
I would watch the patient closely in that situation. If there
were signs of early disease progression, it would not take much
to convince me to change direction and think about using trastuzumab.
Atwo-year time period is the threshold at which point one would
be biased in favor of using more hormone therapy. We know that an
early relapse on adjuvant tamoxifen does not speak well for subsequent
responses to hormone therapy. In some studies, two years has been
the cut-off for the likelihood of response to subsequent hormonal
treatments.
Second-line therapy for recurrent ER-positive,
HER2-positive breast cancer
In a patient not responding to first-line therapy with an aromatase
inhibitor, the algorithm is a little different from what it was
in the past. There are now three options.
The first option is to consider yet another hormonal therapy.
Since the response to one hormonal therapy, on average, predicts
the response to subsequent hormonal therapies, I think that is the
option least likely to be successful. The other options include
chemotherapy, conventionally, and single-agent trastuzumab, less
conventionally.
Choice of therapy for women with indolent disease
Until a few years ago, the only other option would have been chemotherapy.
For a patient with what appears to be indolent disease but that
is nevertheless progressing, the toxicities of many chemotherapy
agents would make me less enthusiastic about this approach. The
availability of single-agent trastuzumab changes the playing field.
In this type of patient, I would feel most justified in using single-agent
trastuzumab.
Randomized trial data clearly shows a time-to-progression and
survival advantage for chemotherapy plus trastuzumab, compared to
chemotherapy alone, and no data demonstrates that trastuzumab alone
is equivalent to trastuzumab plus chemotherapy. There is indirect
data, however, suggesting that trastuzumab can be initiated, and
if there is disease progression, chemotherapy can subsequently be
started while continuing the trastuzumab, without any real loss
of apparent benefit.
From Chuck Vogel’s data there is good evidence that in patients
with HER2- positive (FISH-positive or IHC 3+) metastatic disease,
single-agent trastuzumab before chemotherapy is comparable to conventional
chemotherapy. That data provides me with the basis for using single-agent
trastuzumab.
Choice of therapy for women with visceral crisis
In patients with approaching visceral crisis (i.e., ascites, a
pleural effusion, multiple new pulmonary nodules and multiple new
liver metastases), I would start chemotherapy plus trastuzumab.
If the patient had received adjuvant AC and paclitaxel, in my practice,
the patient would be treated with trastuzumab in combination with
vinorelbine, gemcitabine or often capecitabine.
These are combinations for which there are no randomized trial
data. But the patient is, most likely, refractory to paclitaxel,
which would have normally been my first choice. So, I must base
my decision on Phase II evidence, and for all those drugs, there
is Phase II evidence of safety.
Third-line therapy for recurrent ER-positive,
HER2-positive breast cancer
In patients progressing after one year of trastuzumab, I would
next turn to chemotherapy. One situation where I would almost always
continue trastuzumab would be when I did not think the trial was
adequate. For example, in a patient treated with trastuzumab who
had progression at week eight, I would be concerned that we did
not establish adequate serum levels.
If the patient had not received paclitaxel in the adjuvant setting,
I would use paclitaxel. If the patient had received paclitaxel in
the adjuvant setting, I would use capecitabine next. The quality-of-life
aspects of capecitabine make it a natural choice.
I am biased towards capecitabine because of its oral route, flexible
scheduling/dosing and its decidedly different toxicity profile.
It does not cause profound neutropenia or alopecia. Commonly, patients
who have had adjuvant AC/paclitaxel and develop metastatic disease
are disheartened when they are told that they will get alopecia
once again. In terms of quality of life, being able to offer a regimen
that does not cause alopecia is appealing.
I do not use the package insert dose of capecitabine for any patients
initially. For compliance reasons, I think it is easier to recommend
a single pill size — 500 milligrams. Typically, I calculate
the dose based on a 25% reduction from the package insert dose —
about 1,000 mg/m2 twice a day for 14 days. Going into the second
cycle, I often escalate the dose a bit, maybe by one pill a day,
for patients without any toxicity.
I think that with this adjusted-dose approach, most patients get
away with minimal diarrhea and nominal hand-foot syndrome. When
we first started using capecitabine, we encountered serious diarrhea.
Now that we have made this adjustment, I do not believe we see the
same incidence. Certainly, we do not have the same trouble having
patients continue on the drug that we did at the very beginning
with the full doses. In terms of the hand-foot syndrome, we tend
to dose capecitabine to the point where the hands are a little bit
red.
HER2-positive, hormone receptor-negative inflammatory
breast cancer in an 82-year-old woman
If the patient were healthy enough to have surgery, my inclination
would be to use combination chemotherapy (doxorubicin/cyclophosphamide),
and if she responds, then treat her with surgery followed by paclitaxel
and radiation therapy.
This patient would be also eligible for the CALGB trial evaluating
neoadjuvant trastuzumab. That trial involves four cycles of AC,
with or without dexrazoxane, and 12 weeks of paclitaxel/trastuzumab
followed by surgery and trastuzumab to complete a year. If she were
not healthy enough to have surgery, that changes the equation a
little for me. Then, I would actually be thinking about trastuzumab
for its palliative benefit. I would think of her as a patient with
metastatic disease.
Controversy regarding sentinel node examination
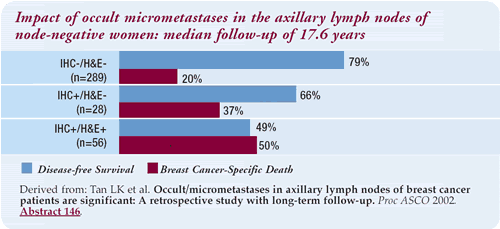
Two abstracts presented at ASCO 2002 highlighted this controversy.
One, a retrospective analysis of a large data-set suggests that
the discovery of any cancer cells (either by IHC or H&E) —
regardless of the number — connotes a poor prognosis compared
to no cells.
Interestingly, the curves do not appear to diverge until many
years — maybe eight or more. If this were the case, it warns
us not to be so quick to accept the lack of significance seen with
short-term follow-up studies. The limitation to this study is that
it was not a sentinel node trial. The patients all had axillary
dissections, which were retrospectively analyzed. Since the vast
majority of patients on this trial had a single positive node, we
think it could correlate to the sentinel node.
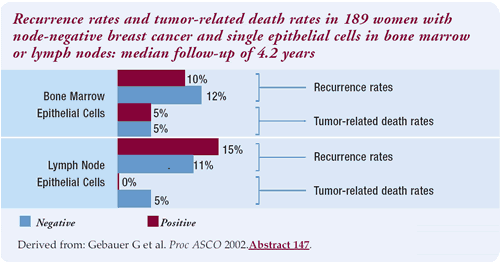
On the other hand, the abstract presented immediately afterwards
suggested no link between survival and the discovery of epithelial-staining
cells in the bone marrow or the sentinel node. The discrepancy highlights
that this is still an unanswered question.
In patients with an IHC-positive sentinel node that is H&E
negative, it is a tough call. Patients with larger tumors with poor
prognostic features will receive treatment anyway. The situations
that are the most difficult are the older patient who will be treated
with hormonal therapy alone, or the patient with a very small primary
tumor for whom presence of nodal involvement would dramatically
change the approach.
Based on the retrospective data-set from our center — presented
at ASCO — I am inclined to think about systemic therapy for
those patients. However, I would prefer that those patients enroll
on a clinical trial.
Select publications
|
