|
You are here: Home: BCU 7|2002:Hyman B Muss, MD
Edited comments by Dr Muss
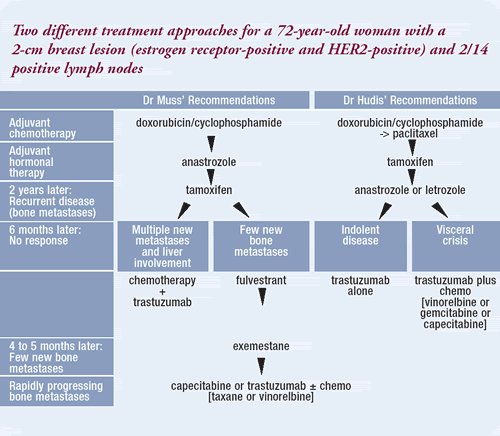
| CASE 2: 72-year-old woman
with ER+ PR+ HER2+ breast cancer |
History
This vigorous, active and very healthy woman presented with
a 2-centimeter tumor that was hormone receptor-positive and
HER2-positive. Two of 14 lymph nodes were positive. Atorvastatin
(Lipitor®) was the only medication she was taking.
Follow-up
She was treated adjuvantly with four courses of doxorubicin/cyclophosphamide
followed by anastrozole, which was well tolerated.
Case discussion
I think the mindset that some physicians have is that 70-year-olds
are “really old,” but a healthy 70-year-old woman
can live another 15 years. A patient with several positive
nodes has a very high risk of recurrence, and breast cancer
may shorten her life.
Selection of adjuvant hormonal therapy
In patients like this one with HER2-positive disease (IHC
3+ or FISH-positive), there are specific reasons to consider
an adjuvant aromatase inhibitor. As suggested by the molecular
biology and preclinical data, women with HER2-positive disease
may have some degree of tamoxifen resistance.
Additionally as patients get older, their risk of vascular
disease generally increases. Tamoxifen probably does not substantially
increase the risk above baseline, but it is a concern.
A positive HER2 status would lead me to consider an aromatase
inhibitor in postmenopausal patients. I would also probably
consider the implications on bone and get a baseline bone
mineral density.
Treatment on relapse
If this patient presented two years later with hip pain,
a bone scan that was positive in several areas, normal laboratory
values and no evidence of other obvious metastases, I certainly
would use another form of endocrine therapy. It would be reasonable
to select tamoxifen, because she has not been exposed to it.
Even though she has HER2-positive disease and that might make
the response rate to tamoxifen lower, I would probably still
try it.
Fulvestrant would also be a reasonable alternative. We really
do not know about the influence of HER2 status on the response
to fulvestrant. Theoretically, it may not be influenced because
of fulvestrant’s different mechanism of action.
There is preclinical data suggesting that trastuzumab can
reverse resistance to endocrine therapy. The CALGB will be
conducting a clinical trial in women who develop a recurrence
while on adjuvant tamoxifen, or shortly after stopping it.
Those women will be randomized to trastuzumab alone or trastuzumab
plus tamoxifen. The trial will determine whether tamoxifen
resistance can be reversed by trastuzumab. There will also
be similar trials with the aromatase inhibitors.
Use of bisphosphonates in patients
with metastatic breast cancer
At the initial diagnosis of metastatic disease to the bone,
I put patients on a bisphosphonate. These agents are all pretty
much equivalent, but I think most of us are using zolendronate
because short infusions are preferred over long infusions.
There are differences in the potency of the bisphosphonates,
but there are no convincing differences in their efficacy
when used at the optimal doses.
Second-line therapy for recurrent breast
cancer
In the first few months, endocrine therapy can produce a
flare in markers, alkaline phosphatase, skin lesions and bone
pain. If after six months of tamoxifen, however, this patient
was having more hip pain and the bone scan had a few more
lesions, I believe I would try further endocrine therapy if
the symptoms were modest. Since chemotherapy is palliative,
there is no rush to use it.
On the other hand, if the patient had a profound change
in tumor biology, multiple new metastases, pain all over and
substantial liver involvement, I would probably turn to chemotherapy.
In the typical patient, however, the scenario is slower, and
there is time to act. Therefore, I would try another endocrine
therapy.
Fulvestrant would be a very good choice. One could also
try a progestin, but I am impressed by the data comparing
fulvestrant to anastrozole in patients who are refractory
to tamoxifen. It is fascinating that fulvestrant, which prevents
dimerization of the estrogen receptor, attacks the same estrogen
receptor that was not effectively using tamoxifen as an antagonist.
This means that the cancer is still endocrine-dependent.
Third-line therapy for recurrent breast
cancer
If after four to five months of fulvestrant, the patient
then developed a few more lesions but was still in pretty
good shape, it would be reasonable to consider another aromatase
inhibitor, such as exemestane. In a study by Lonning et al,
exemestane was effective in women who were refractory to either
letrozole or anastrozole.
Fourth-line therapy for recurrent breast
cancer
If the patient’s bone metastases were rapidly progressing
every few months, I think it would be reasonable to next use
trastuzumab alone, trastuzumab plus a taxane, or perhaps trastuzumab
plus vinorelbine. It would be rational to select a drug like
capecitabine as well.
If the patient was not very symptomatic, I suspect there
would not be great differences with any of those interventions.
The response rates will certainly be higher, I suspect, for
trastuzumab plus a taxane than for capecitabine. My personal
approach, though, has been to use capecitabine in many of
these patients.
If the patient certainly had any major progression, my bias
would be to give trastuzumab plus chemotherapy. If the patient
were somewhat reluctant about chemotherapy for any reason,
single-agent trastuzumab would be an alternative. In Vogel’s
paper, the time to progression and survival for single-agent
trastuzumab was similar to those in the pivotal trial. Although
it is not a fair comparison, the numbers look pretty good.
I am sensitive to the data from the trastuzumab pivotal
trial, which demonstrated a survival advantage of about five
months for trastuzumab plus chemotherapy compared to chemotherapy
alone. The pivotal trial was not designed so that trastuzumab
was given to all patients secondline after chemotherapy, which
would have conclusively proven that there was an advantage
to giving trastuzumab plus chemotherapy, together. I suspect
I am a “contrarian” here.
| Fulvestrant
versus anastrozole: Conclusions |
“These data
demonstrate that fulvestrant is the first antiestrogen
to show comparable efficacy to anastrozole in the second-line
treatment of advanced breast cancer. These data also
confirm previous findings of the phase II study that
a pure antiestrogen is effective in tamoxifen-resistant
patients...Overall, both treatments were well tolerated,
with the percentage of patients experiencing adverse
effects (AE) being similar and few patients withdrawing
because of AEs. Most events were mild and the safety
profiles were similar. The most frequently reported
drug-related AEs were hot flashes, nausea, sweating,
headache, and asthenia; all are recognized side effects
of endocrine treatments for breast cancer.
Fulvestrant is given as an IM injection once a month.
Injection site reactions were uncommon and mild in intensity,
with only one reaction leading to a patient withdrawing
from treatment, thus demonstrating that the use of the
fulvestrant injection is well tolerated.
Taken overall, these data demonstrate that fulvestrant
is as effective as anastrozole, with similar tolerability
and QOL effects. Fulvestrant should offer clinicians
a new option for the treatment of postmenopausal women
with advanced breast cancer whose disease progresses
after tamoxifen treatment.” |
| Howell A et al.
Fulvestrant, formerly ICI 182,780, is as effective
as anastrozole in postmenopausal women with advanced breast
cancer progressing after prior endocrine treatment. J
Clin Oncol 2002;20:3396-403. Abstract |
| Combined
analysis of two studies evaluating fulvestrant (FAS) versus
anastrozole (AN) in tamoxifen resistant patients with
metastatic disease |
". . . all
patients were included in a newly developed statistical
analysis of DOR, defined for responders as the time
from onset of response to disease progression and for
nonresponders as zero. In this analysis, DOR was significantly
greater (ratio of average response durations = 1.30;
95% CI 1.13 to 1.50; p=0.0003) for FAS vs AN. It can
therefore be concluded that FAS is at least as effective
as AN and provides prolonged DOR in postmenopausal women
with ABC." |
| EXCERPT
FROM: Parker LM et al. Greater duration
of response in patients receiving fulvestrant ('Faslodex')
compared with those receiving anastrozole ('Arimidex').
Proc ASCO 2002;Abstract
160. |
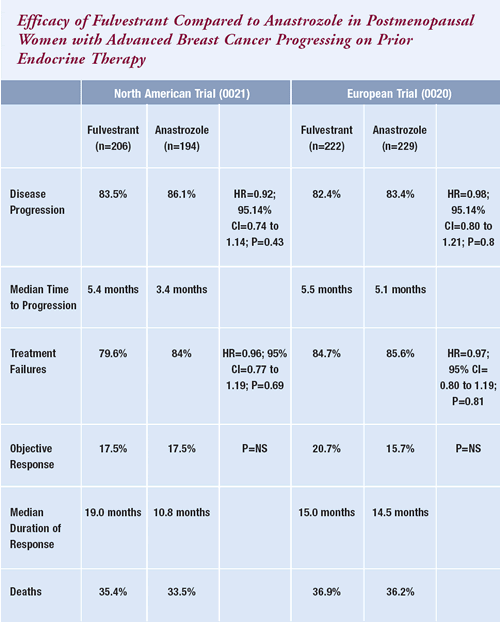
* In those responding to treatment.
Derived from Osborne CK et al. Double-blind, randomized
trial comparing the efficacy and tolerability of fulvestrant
versus anastrozole in postmenopausal women with advanced breast
cancer progressing on prior endocrine therapy: Results of
a North American trial. J Clin Oncol 2002;20:3386-95.
Abstract
Howell A et al. Fulvestrant, formerly ICI 182,780,
is as effective as anastrozole in postmenopausal women with
advanced breast cancer progressing after prior endocrine treatment.
J Clin Oncol 2002;20:3396-403. Abstract |
82-year-old woman with inflammatory breast cancer
I just evaluated an 82-year-old woman with HER2-positive, estrogen
receptornegative inflammatory breast cancer. She was in excellent
condition with minimal other medical problems. I treated her very
aggressive tumor with a taxane and trastuzumab, which she tolerated
superbly and to which she had a fantastic response. Now, I am treating
her with trastuzumab every three weeks. Although she is not on a
clinical trial, she did have inflammatory breast cancer with some
positive nodes after surgery.
There is no evidence to suggest that trastuzumab’s tolerability
or efficacy is any different in the elderly. I have actually treated
several very elderly women with trastuzumab.
Hormonal therapy versus chemotherapy for women
with metastatic breast cancer
In metastatic disease, sequential chemotherapy is as good as combination
chemotherapy. The majority of studies have shown no improvement
in survival for more intense over more modest regimens.
I believe the goal for the patient with metastatic breast cancer
is controlling the disease by preventing tumor growth for as long
as possible. In patients with mild bone pain, keeping them stable
for five years without any tumor shrinkage, and being able to manage
their pain with an anti-inflammatory, will maintain their quality
of life better than aggressive chemotherapy that may shrink the
tumor but cause more toxicity. I believe in sequential therapy,
which is associated with less toxicity.
Chemotherapy selection for patients with metastatic
breast cancer: Role of capecitabine
In patients with lymphangitic spread or a bilirubin of 3 mg/dL,
time is of the essence, and I would select an anthracycline, a taxane
or capecitabine/ docetaxel. But, those are the minority of patients.
The goals of treatment are disease control — providing symptoms
are modest — and quality of life.
I use a lot of single-agent capecitabine. In two small randomized
Phase II trials, which should really not be compared, the response
rates are similar to CMF or paclitaxel. Additionally, capecitabine
is an oral agent, and it does not cause hair loss. Many patients
have had prior adjuvant chemotherapy, and they may have had bad
experiences from previous hair loss.
Capecitabine is an extremely well tolerated drug. It is rare to
see myelosuppression with capecitabine. If a patient does not have
hand-foot syndrome, they will probably tolerate it very well. I
think that diarrhea is generally modest, but the hand-foot syndrome
can be substantial.
I suspect that the dose in the package insert is too high. Data
suggests that doses of 2,000 or perhaps 1,500 mg/m2/day (in two
divided doses) for 14 consecutive days are as effective. The incidence
of the hand-foot syndrome declines substantially with these doses,
and it becomes necessary to reduce the dose in only about 15% of
patients.
Adjuvant chemotherapy for elderly women
In unpublished data from the Oxford 2000 Overview, there were
about 1,200 elderly patients (=70 years old), out of about 200,000,
who were randomized to adjuvant chemotherapy or observation. The
proportional reduction in the risk of relapse associated with adjuvant
chemotherapy was very similar for that group of patients. In fact,
it was a bit higher than that for the 60- to 69-yearold group, and
similar to that for the 50- to 59-year-old group.
This data suggests that there is no reason that the Overview,
which supports the value of adding adjuvant chemotherapy to endocrine
therapy, would not apply to older women. The question is, “Is
the patient going to live long enough to obtain a benefit?”
In the United States, a 70-year-old woman in fair health will live
on average to 85 years of age. A 75-year-old woman will also live
to about 86 or 87 years of age, and an 80-year-old woman will live
an average of another six to eight years. Seventy-year-old patients
have a 15-year life span. If they have three or four positive lymph
nodes or a very large, highgrade, node-negative tumor and are in
reasonable health, breast cancer will be the major problem in their
life.
CALGB: Adjuvant trial of capecitabine versus
CA/CMF
In my adjuvant trial for elderly (= 65 years) women with node-positive
breast cancer or high-risk (= 3 cm), node-negative breast cancer,
patients are randomized to either standard chemotherapy or capecitabine.
Because of the controversies about standard therapy, we gave doctors
and patients the option of either CMF with an oral cyclophosphamide
regimen or AC.
We have a quality-of-life assessment as part of the trial. We
are looking at function and comorbidities, a major issue in the
management of older women with breast cancer in the adjuvant setting.
We are also going to evaluate other issues including the biology
of breast cancer and patient compliance. In a companion study with
tissue blocks, we will look at HER2 and thymidine phosphorylase,
which is related to the effect of capecitabine. This trial in older
women may provide clues on how to predict which patients will benefit
from what therapies.
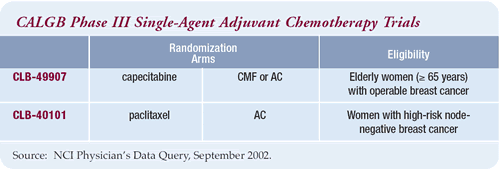
CALGB Phase III Single-Agent Adjuvant Chemotherapy
Trials
The Overview demonstrated that combination chemotherapy was better
than single-agent chemotherapy in the adjuvant setting. These results,
however, were based on single-agent trials with older drugs like
melphalan. In fact, we are resurrecting single-agent chemotherapy
in the adjuvant setting through our trial in older women, as well
as a large CALGB trial in women with nodenegative disease.
CALGB node-negative trial
The CALGB trial in women with node-negative breast cancer will
compare weekly paclitaxel with doxorubicin/cyclophosphamide as adjuvant
therapy. It has a two-by-two factorial design. Patients randomized
to doxorubicin/ cyclophosphamide will receive either four or six
cycles, and patients randomized to paclitaxel will receive either
12 or 18 weeks of therapy. This study is based on some randomized
neoadjuvant trials conducted at MD Anderson, which compared paclitaxel
to FAC.
Select publications
|
|
