| You are here: Home: BCU 5|2002: Eleftherios P Mamounas, MD
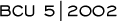
Adjuvant trial of capecitabine as therapy for
elderly women
Hy Muss is conducting an adjuvant trial comparing either AC or
CMF to capecitabine in elderly women. We do not have convincing
data that adjuvant chemotherapy provides a benefit to women over
the age of 70. In the Overview Analysis, there was a decrease in
the risk reduction with every decade of life. However, there were
few women over the age of 70 included in that analysis. Today, many
women over the age of 70 have a good performance status and no comorbid
conditions.
Yet there is still reluctance, especially in women with ER-positive
tumors who will receive adjuvant tamoxifen or perhaps anastrozole,
to use adjuvant chemotherapy. We actually need to compare adjuvant
hormonal therapy with or without chemotherapy in elderly women with
ER-positive breast cancer. If chemotherapy improves survival, then
we can look for regimens that are equally effective and less toxic.
In women with ER-negative breast cancer, most physicians are comfortable
using adjuvant chemotherapy. Therefore, Hy Muss’ trial may
be more applicable to elderly women with ER-negative breast cancer.
Adjuvant trastuzumab in HER2-positive, node-positive
disease
NSABP B-31 evaluates adjuvant trastuzumab in women with HER2-positive,
node-positive breast cancer. It is a two-arm trial comparing doxorubicin/
cyclophosphamide followed by paclitaxel with or without trastuzumab.
As demonstrated by the trials in metastatic disease, trastuzumab
cannot be given concomitantly with anthracyclines because of the
potential cardiac toxicity. We are evaluating whether they can be
given sequentially. B-31 has very strict criteria for the evaluation
of cardiac toxicity. An interim analysis conducted by the Data Safety
Monitoring Committee did not find a significant incidence of cardiac
toxicity associated with the addition of trastuzumab. In contrast,
the NCCTG, because of cardiac toxicity, had to suspend the arm of
trial 9831 that administered concomitant trastuzumab and paclitaxel.
In B-31, we conducted an analysis of the first 100 patients’
HER2 assays. There was significant inconsistency between the HER2
results obtained from nonreference laboratories and those obtained
with FISH at a central laboratory. The protocol now mandates that
a reference or central laboratory perform the IHC assay before the
patient is enrolled on the trial.
In the metastatic setting, trastuzumab in combination with chemotherapy
prolongs median survival by five months. We hope that adjuvant trastuzumab
will also be of benefit. In light of the cardiac toxicity, we must,
however, be careful not to adopt trastuzumab into the adjuvant setting
before we have results from randomized clinical trials. Theoretically,
one could justify using trastuzumab in a woman with inflammatory
breast cancer; I would still be very hesitant without proven benefit,
especially in lieu of the side effects.
Adjuvant clodronate
NSABP B-34 will evaluate adjuvant clodronate, an oral bisphosphonate,
in women with node-negative and node-positive breast cancer. Data
from Germany as well as the Canadian and UK trials demonstrate that
clodronate reduces bone and non-bone metastases and also improves
survival. B-34 will randomize 3,000 women to three years of clodronate
or placebo. The choice of adjuvant therapy will be left to the investigator’s
discretion. We chose clodronate for this trial because it is the
only bisphosphonate with data in the adjuvant setting.
Clodronate is well tolerated compared to alendronate (Fosamax®).
If the B-34 results are positive, hopefully clodronate will be FDA
approved. In lieu of the ATAC trial results, it may be reasonable
to combine an aromatase inhibitor with a bisphosphonate as adjuvant
therapy. Eventually, NSABP plans to compare the bisphosphonates
to find the best one. It may, however, be difficult to use an intravenous
bisphosphonate in terms of patient acceptability.
Anastrozole in women with DCIS
We are close to initiating a trial in women with DCIS that will
compare anastrozole to tamoxifen. This trial will replicate the
ATAC trial in women with DCIS. Since these are very low-risk women,
it is important to determine whether the risk-benefit ratio will
justify the use of an aromatase inhibitor. The additional 50% reduction
in contralateral breast cancer, associated with anastrozole in the
ATAC trial, justifies the design of this trial in women with DCIS.
Implications of the ATAC trial results
This is the first time that an agent appears to be superior to
tamoxifen. Even though the disease-free survival and contralateral
breast cancer data are promising, it is still not known whether
this will translate into a survival advantage. In elderly patients
in whom one wants to avoid thromboembolic events and endometrial
cancer, the ATAC trial results may lead to the adoption of anastrozole
as adjuvant therapy. Eventually, anastrozole may be used in most
postmenopausal women. In terms of the ongoing NSABP trials, we currently
only allow the use of tamoxifen. However, that may have to change.
For our new trials, the NCI is already stating that we must allow
the use of an aromatase inhibitor. In Europe, the IBIS trial will
compare tamoxifen to an aromatase inhibitor as preventative therapy.
Proposed trial of XT in ipsilateral breast tumor
recurrence or local regional recurrence
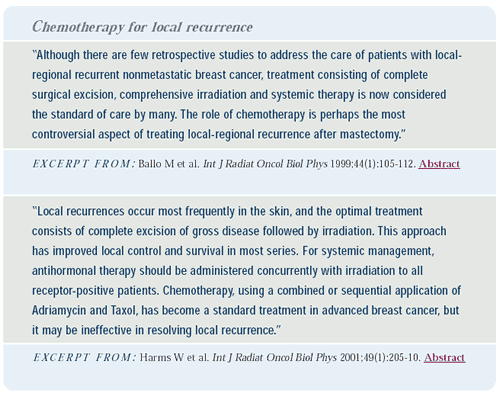
We are planning a trial to determine the value of chemotherapy
in patients with ipsilateral breast tumor recurrence (IBTR) or local
regional recurrence. These patients have not been studied prospectively,
and it is not known whether chemotherapy can improve survival. Patients
with IBTR and local regional recurrence have a 50%-60% and 80%-90%
risk respectively, of developing systemic disease in the next five
years. In ER-positive patients, we will compare hormonal therapy
with or without capecitabine/docetaxel. In ER-negative patients,
we will compare capecitabine/ docetaxel to no treatment. Since most
of the patients will have received either an anthracycline or alkylating
agent as adjuvant therapy, we chose docetaxel as a noncross-resistant
agent. Capecitabine was added to obtain the maximum benefit.
Select Publications
Page 2 of 2
|
