|
You are here: Home: BCU 4|2002: Eric P Winer, MD
Edited comments by Dr Winer
Less toxic regimens for metastatic breast cancer
Throughout the 1990s, the focus in breast cancer research was on high-dose chemotherapy and dose-intensive regimens. Clearly, the pendulum has now swung in a different direction. Over the course of the last decade, we have learned that more is not necessarily better. There has been tremendous interest in identifying more tolerable regimens that may also be more effective, with a focus on targeted therapies.
We have conducted a number of trials taking advantage of oral dosing schedules and low-dose weekly schedules. These trials not only allow women to have their cancer under control but also to lead as normal a life as possible. First and foremost, we would like to cure every woman’s breast cancer, but if we cannot do that, then the least we can do is help women live longer without suffering severe treatment-related toxicities.
We have come a long way in terms of making life more tolerable for women with advanced breast cancer. Not long ago, patients would spend much more time in the hospital either receiving chemotherapy or recovering from its toxicities. We have moved away from that type of approach. Although a small number of women may benefit from high-dose chemotherapy, studies have not demonstrated an overall benefit. In fact, single-agent chemotherapy may be as effective as combination chemotherapy.
Of course, some combination chemotherapy regimens are associated with higher response rates, but sequential single-agent chemotherapy is probably associated with the same overall survival. Single-agent chemotherapy allows us to tailor the treatment to the individual woman. The ability to combine the newer biologic agents with single-agent chemotherapy is another advantage.
Quality and duration of life in metastatic breast cancer
In the treatment of women with metastatic breast cancer, the two most important considerations are the quality and duration of life. For those receiving chemotherapy, their quality of life is a balance between disease control and treatment-related toxicity. In women with tumor-related symptoms, quality of life is mainly determined by tumor control. In the capecitabine/docetaxel (XT) trial, a survival benefit was associated with the XT combination compared to docetaxel alone; however, relatively few patients in the docetaxel arm later received capecitabine. To me, what this trial demonstrates is that capecitabine is an excellent agent for breast cancer.
Capecitabine in clinical practice
I use a lot of single-agent capecitabine fairly early in the management of women with metastatic breast cancer. In fact, outside of a clinical trial, I almost exclusively use it as a single agent. In metastatic disease, there is no specific order in which to give chemotherapeutic agents. Since women stay on their first- or second-line treatments longer than their third- and fourth-line treatments, it makes sense to use the more tolerable agents earlier.
Therefore, outside of a clinical trial, I frequently give capecitabine as either first- or second-line therapy. In order to minimize toxicities, I usually start with a dose of 2,000 mg/m2 in two divided doses for two weeks followed by one week off therapy. There is concern about the hand-foot syndrome and diarrhea associated with capecitabine, but if one is attentive to the dose and educates patients about stopping treatment if they experience side effects, it is very well tolerated.
| Capecitabine and quality of life |
| In addition to its role in taxoid-resistant breast cancer, capecitabine may be useful as second-line therapy in anthracycline-resistant metastatic breast cancer or as first-line treatment as an alternative to intravenous chemotherapy. The improvement of patients’ quality of life achieved by using oral agents with similar efficacy but enhanced tolerability is a vital component of the care of cancer patients where the goal of treatment is palliation. Furthermore, the low incidence of myelosuppression makes capecitabine an attractive agent for use in the adjuvant setting and also for incorporation into combination regimens, either with conventional agents, for example epirubicin/doxorubicin, or agents such as the taxoids and vinorelbine. |
| EXCERPT FROM: Leonard RCF. Br J Cancer 2001;84(11):1437-42. Abstract |
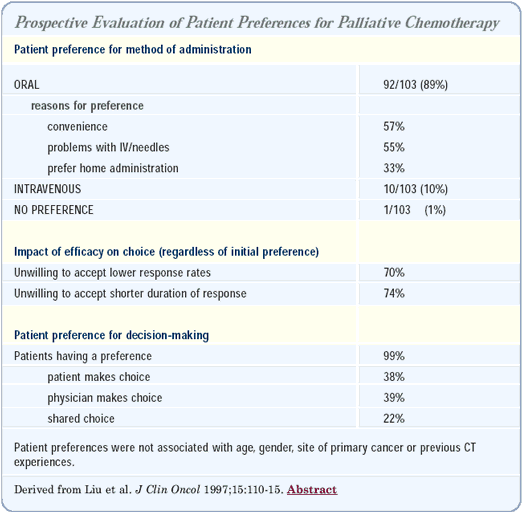
Patient preference for oral versus intravenous chemotherapy
As long as it does not increase toxicity or compromise efficacy, most patients prefer the oral route of administration. Geoffrey Liu conducted a patient preference study, which questioned patients about their preferences for oral or intravenous chemotherapy. If it provided similar efficacy and fewer side effects, 90% of the patients would choose oral chemotherapy.
First-line chemotherapy in women who have received an anthracycline as adjuvant therapy
Outside of a clinical trial, a woman who has received an anthracycline as adjuvant therapy could potentially receive either docetaxel, paclitaxel, capecitabine or vinorelbine as first-line therapy for metastatic disease. In my opinion, the response rates for these agents are fairly similar. Some believe docetaxel is the most active agent, but I am not convinced that any of these agents have different activity. I tailor the treatment to the woman and base my decision on the types of side effects the woman would prefer to avoid.
From a toxicity standpoint, the best agents are probably capecitabine and vinorelbine. Alopecia is often an issue for women, and capecitabine is not associated with hair loss. If one is careful with the capecitabine dose, most side effects can be avoided. Over time, some women may experience chronic changes in their hands and feet, but that is the predominant toxicity encountered with capecitabine. In women without rapidly progressive disease, I frequently use capecitabine.
| Choice of systemic agents for metastatic breast cancer |
| Until recently, the treatment alternatives to taxoids were very limited. However, results from studies in advanced breast cancer of the new, oral, enzymatically activated fluoropyrimidine, capecitabine, indicate that this agent may be useful when anthracycline-based chemotherapy fails as well as in taxoid failures. Capecitabine may offer an effective, well-tolerated and more convenient alternative to taxoids and other intravenous cytotoxic agents. Clinical trials of capecitabine in breast cancer have demonstrated substantial activity with durable responses and meaningful clinical benefits. |
| EXCERPT FROM: Leonard RCF. Br J Cancer 2001;84(11):1437-42. Abstract |
Rapidly progressing metastatic breast cancer
Combination chemotherapy may make more sense in this situation, particularly if I believe that the woman realistically has only one chance to improve. This is also the sole situation in which I would combine chemotherapy with hormonal therapy for a woman with estrogen receptorpositive disease. Although I may have concerns about combination chemotherapy in a woman with a poor performance status, I would probably use it. This is actually the type of situation where I would consider agents like doxorubicin, the taxanes or the capecitabine/docetaxel combination.
Management of women with metastatic hormone receptor-positive breast cancer
Since hormonal therapy is better tolerated than chemotherapy, most women with metastatic hormone receptor-positive breast cancer should initially receive hormonal therapy. Responses to hormonal therapy are often durable, and women are usually better off waiting to receive more toxic therapy.
There are exceptions to this rule, especially in women with very rapidly or moderately rapidly progressing disease. These women have a below average chance of responding to hormonal therapy. In the past, it was speculated that it might take longer to respond to hormonal therapy than chemotherapy.
Perhaps chemotherapy provides a little faster response than hormonal therapy, but we probably made that more of an issue than necessary.
It may be time to challenge the assumption that the presence of visceral metastases decreases the likelihood of responding to hormonal therapy. Visceral metastases in 2002 are quite different than those in 1975. In 1975, a woman who had liver metastases often had a very large liver, abnormal liver function tests and elevated bilirubin. In 2002 many of those lesions are seen on a spiral CT, and frequently these may be 1-2 centimeters, entirely asymptomatic and with normal liver function tests.
Management of women with metastatic HER2-positive breast cancer
Even though there is suggestive evidence that women with HER2-positive, ER-positive breast cancers may be less likely to respond to hormonal therapy, I would still consider using it. When it is time to advance to chemotherapy in women with HER2-positive breast cancer, trastuzumab is the standard of care. Whether or not to combine trastuzumab with chemotherapy is the only remaining question. Since the response rates and the control of tumor-related symptoms are higher for trastuzumab plus chemotherapy, oncologists commonly administer the combination. Additionally, the survival benefit seen with trastuzumab in the pivotal trial by Slamon and colleagues was obtained when chemotherapy and trastuzumab were given together.
Trastuzumab monotherapy
There are a few situations in which trastuzumab may be used alone. First is the woman who wishes to avoid chemotherapy-related side effects. The second situation involves the woman with fairly minimal disease or disease that is progressing slowly. Another situation involves the woman who received adjuvant AC/paclitaxel and three months later has recurrent disease. This woman has demonstrated fairly chemotherapy-resistant disease, and her chance of improving is dependent upon her response to trastuzumab. It may be worth thinking about trastuzumab alone in this type of woman, although in the end I probably would also use chemotherapy.
Trastuzumab plus chemotherapy
For the time being, trastuzumab should not be given with an anthracycline because of the potential cardiotoxicity. The standard of care is trastuzumab plus paclitaxel. Given the activity of docetaxel in women with metastatic breast cancer and the potential preclinical synergy, there are many physicians who administer trastuzumab plus docetaxel.
Approximately three years ago, we started studying trastuzumab plus vinorelbine. In our first phase II study with 40 women, trastuzumab plus vinorelbine was well tolerated with an overall response rate of 75%. There is an on-going multicenter phase III trial, with 50 sites in the United States, comparing vinorelbine/trastuzumab to a taxane/trastuzumab regimen.
Vinorelbine has not traditionally been considered a first-line agent for the treatment of metastatic breast care; however, vinorelbine/trastuzumab is a promising regimen. I predict that the efficacy between the two arms will be fairly similar, and the toxicity with the vinorelbine/trastuzumab arm will be lower.
| Trastuzumab/vinorelbine for HER2-overexpressing metastatic breast cancer |
| The overall response rate was 75%, with a suggestion of higher response rates observed in subgroups of patients with HER2 +3 positive tumors, and among patients receiving the combination regimen as first-line chemotherapy for metastatic breast cancer. Response rates in excess of 60% were observed for patients receiving the regimen as second- or third-line therapy for metastatic cancer, and among patients who had peviously received anthracycline- and taxane-based chemotherapy. Acute toxicities were quite mild and manageable. Neutropenia was the most common severe toxicity and was managed with vinorelbine dose modification without other sequelae. Gastrointestinal side effects and alopecia were modest. Sustained therapy with vinorelbine and trastuzumab was feasible without encountering cumulative side effects. A small percentage of patients were taken off study for asymptomatic declines in left ventricular EF. |
| EXCERPT FROM: Burstein HJ et al. J Clin Oncol 2001;19(10):2722-30. |
There are both US and European trials evaluating the combination of capecitabine and trastuzumab, which could potentially be a well-tolerated regimen. Anecdotally, I have seen a number of good responses to singleagent capecitabine in women with HER2-positive disease who have failed trastuzumab.
Therapy for women progressing on a trastuzumab/chemotherapy regimen
Although I sometimes continue trastuzumab after a woman progresses, there is no clear evidence that it is beneficial. MD Anderson is conducting a trial to compare vinorelbine to vinorelbine/trastuzumab in women who progress after taxane/trastuzumab combination therapy.
In the nonprotocol setting for a woman who progresses on trastuzumab, I tend to use it once more. Women often feel very strongly about continuing trastuzumab, particularly if they have had a response. In patients who have responded to a trastuzumab-containing regimen, at the time of disease progression, I typically try one more trastuzumab-containing regimen or stop the trastuzumab and then come back to it later on.
One exception is the woman who responds to a trastuzumab-containing regimen and then develops CNS metastases. I consider that woman to have progressive disease in the brain but not trastuzumab-refractory disease. Therefore, I would continue the same regimen and treat her CNS disease with cranial irradiation.
| Serum versus CSF levels of trastuzumab |
| It is unknown whether and to what extent trastuzumab can cross the blood-brain barrier. Therefore, we measured CSF and concomitant serum levels of trastuzumab in a 62-year-old patient with meningeal carcinomatosis treated with weekly intravenous trastuzumab. A few hours after trastuzumab infusion, serum levels achieved were as expected in the range of 10,000 to 100,000 ng/mL. Concomitant CSF levels were 300-fold lower. Despite a possibly leakier blood-brain barrier in this patient with meningeal carcinomatosis, only minimal amounts of trastuzumab penetrated the CSF. Therefore, it is unlikely that intravenous trastuzumab would be useful to treat meningeal or cerebral disease of breast cancer. |
| EXCERPT FROM: Pestalozzi BC, Brignoli S. J Clin Oncol 2000;18(11):2350-51. |
| CNS progression during a phase II study of docetaxel/trastuzumab in HER2-positive metastatic breast cancer |
| Three patients had CNS progression after an initial response to therapy. These patients were withdrawn from the study. In two of these patients, the CNS was the first and only site of disease progression. These patients continued to receive trastuzumab therapy after wholebrain irradiation and remained without evidence of other systemic progression outside the CNS for 4 and 12 months. The CNS seems to be a sanctuary for disease in patients treated with trastuzumab and docetaxel therapy. This could be due in part to the low penetration of trastuzumab and the taxanes into the brain. |
| EXCERPT FROM: Esteva FJ et al. J Clin Oncol 2002;1800-1808. |
Trastuzumab schedule
Although the approved schedule is weekly, Canadian trials have evaluated an every-three-week schedule both as a single-agent and in combination with paclitaxel. There also has been discussion about changing the Intergroup adjuvant trial to an every-three-week schedule.
When combined with chemotherapy, I still administer trastuzumab on a weekly schedule. In order to make life easier, I have used the every-threeweek schedule for a few women receiving trastuzumab alone, and it has been well tolerated. We will probably see new regimens with an every-three-week schedule for chemotherapy and trastuzumab.
| Schedule of trastuzumab |
| The half-life of trastuzumab seems to be longer than originally reported, and studies are underway to define it accurately. A longer half-life means that dosing less frequently than every three weeks may also be feasible. To this end, modelling based on known pharmacokinetic data is being used to assess the feasibility of novel dosing regimens. This process aims to identify regimens involving the administration of trastuzumabloading doses on consecutive days to generate therapeutic concentrations within a shorter time. Furthermore, the longer half-life means that longer drug-free intervals (six or eight weeks) may be possible between maintenance doses. For example, a regimen involving the administration of trastuzumab 8 mg/kg on three consecutive days followed by 8 mg/kg every six weeks may be feasible. The associated greater dosing flexibility is predicted to improve management of patients further by offering more choice and convenience, including drug holidays. |
| EXCERPT FROM: Leyland-Jones B. Lancet Oncol 2002;3(3):137-44. Abstract |
The patient-physician relationship
When patients are sick and need more medical care, the relationship with their doctor, nurse and social worker becomes more important in terms of quality of life. I try to make decisions with the patients and to provide them with options so they can guide me. Decision-making is about describing the options, knowing your patient and then trying to pick the best option together. Communication is very important. The challenge we all face is being realistic so that patients do not have unrealistic expectations but also have a real sense of hopefulness.
| Patient-physician decision-making in breast cancer |
| Despite the increase in consumerism, many breast cancer patients come to consultations with little or no prior knowledge of breast cancer and want their physicians to choose on their behalf. In breast cancer, many alternatives do not differ in their impact on survival and recurrence but do have very different side effects. In adjuvant therapy, some patients must trade-off the long-term effects of chemotherapy (e.g., infertility and so on) with a small survival difference. Physicians who assume the responsibility for choosing on behalf of their patients need to be able to understand their patients’ preferences. With limited time and resources, physicians need to synthesize their patient’s detailed medical history along with the relevant evidence from the literature and incorporate their patients’ preferences. This is a challenging task to complete in a short visit, even for the most skilled practitioners.
Physicians need to be prepared to handle the diversity of their patients. In particular, physicians need to be able to engage and empower their patients to participate in the consultation in whatever manner is most comfortable for the patient. |
| EXCERPT FROM: Sepucha KR et al. J Clin Oncol 2000;18(6):1230-8. |
ATAC trial results
I was surprised by the ATAC trial results, since I was predicting the three arms, in short-term follow-up, would be equivalent with perhaps differences in toxicity. Based on studies in postmenopausal women with advanced disease, the aromatase inhibitors may be better than tamoxifen. On preliminary and early follow-up, the ATAC trial would also suggest that anastrozole is better than tamoxifen or the combination in terms of diseasefree survival.
Applying these results to clinical practice will be a challenge. In women receiving tamoxifen for more than one or two months, I would not switch to an aromatase inhibitor. Whether I will use anastrozole in every postmenopausal woman in whom I would have given tamoxifen is the pressing issue. In terms of selecting an aromatase inhibitor in the adjuvant setting, I am data-driven, and at this point in time I would use the results of the ATAC trial and prescribe anastrozole. Undoubtedly, there will be much discussion about the applicability of these results to the other aromatase inhibitors.
Select publications
|
|
