| You are here: Home: BCU 4|2002: Aman Buzdar, MD
 |
 |
 |
 |
 |
Aman Buzdar, MD |
 |
 |
Professor of Medicine
Ashbel Smith Professor, Deputy Chairman,
Breast Medical Oncology
University of Texas MD Anderson Cancer Center
Committee Chair, Institutional Review Board
University of Texas MD Anderson Cancer Center |
|
 |
 |
 |
Edited comments by Dr Buzdar
ATAC trial
The standard adjuvant hormonal therapy, tamoxifen, has been very effective in reducing the breast cancer recurrence rate. The main objective of the ATAC trial was to compare the efficacy and safety of adjuvant anastrozole, which lowers estrogen levels, and tamoxifen. A second objective of this trial was to determine if the combination of anastrozole plus tamoxifen would further reduce the recurrence risk or alter the safety profile associated with the individual agents.
Overall efficacy
After two and one-half years of follow-up, the ATAC trial demonstrated that anastrozole further reduced the risk of recurrence compared to tamoxifen. The estrogen receptor status was known for 83% of the women. In estrogen receptor-positive women, there was a 22% reduction in the risk of recurrence with anastrozole compared to tamoxifen. For the group as a whole — including estrogen receptor-negative and receptor-unknown patients — there was a 17% reduction in the risk of recurrence with anastrozole relative to tamoxifen.
Additionally, there was a 58% reduction in the risk of contralateral and ipsilateral breast cancers with anastrozole compared to tamoxifen. There was no additional reduction in the risk of recurrence for the combination of anastrozole and tamoxifen compared to tamoxifen alone. My personal bias is that eventually there will be an increase in survival associated with anastrozole.
Adjuvant anastrozole in women receiving chemotherapy
Approximately 20% of the women in the ATAC trial received adjuvant chemotherapy. The question remains as to whether these women will derive similar benefits from anastrozole. Participation in the trial was delayed until these women finished their systemic chemotherapy. We are now evaluating that subpopulation in more detail. I do not think chemotherapy negates anastrozole’s effects.
Anastrozole plus tamoxifen combination
ATAC was an elegant study, and if there was going to be any synergistic or additive benefit to combined hormonal therapy in breast cancer, this was an ideal setting. The combination arm was slightly inferior to tamoxifen and clearly inferior to anastrozole. Tamoxifen totally negated the effects of anastrozole. For estrogen-dependent tumors in an estrogen-deprived environment, it has been speculated that tamoxifen acts like an agonist.
Overall safety profiles
Tamoxifen, because of its agonistic properties, increases the risk of thromboembolic events, vaginal spotting and bleeding, vaginal discharge and endometrial cancer. All of these side effects were less common with anastrozole than tamoxifen. Compression fractures of the spine, wrist fractures and rib fractures occurred more frequently with anastrozole than tamoxifen.
On the other hand, the risk of hip fractures was identical for anastrozole and tamoxifen. There was also a slight increase in the occurrence of arthralgias associated with anastrozole. In the metastatic setting, only an occasional woman will have discomfort requiring an NSAID. I have not seen any women in whom the arthralgias required a change or discontinuation of therapy. The anastrozole plus tamoxifen combination did not modify the safety profile of these agents compared to tamoxifen alone.
Bone density
A subprotocol of the ATAC trial is systematically evaluating the turnover of bone markers and the changes in bone density. This data will be available in the next few months. Since anastrozole further reduces estrogen levels in postmenopausal women by 95-98%, I personally believe that anastrozole increases bone loss beyond that which normally occurs in postmenopausal women.
Although this is a real side effect, you can monitor women for changes in bone density and institute therapeutic interventions when needed. In my clinic, many women are already on a bisphosphonate — calcitonin or calcium supplements to reduce the risk of osteoporosis. When prescribing anastrozole as adjuvant therapy, baseline and periodic bone densities are indicated. If there is a decrease in bone density, it would be appropriate to begin interventional therapy.
Weight gain
There was less weight gain associated with anastrozole than tamoxifen. Data from NSABP P-1 did not show any change in weight for tamoxifen compared to placebo. The ATAC trial, however, demonstrated some weight gain associated with tamoxifen and no substantial change in weight associated with anastrozole. A majority of women with breast cancer — even those receiving chemotherapy — gain some weight.
Interchangeability of the aromatase inhibitors
A very important question that needs to be addressed is the interchangeability of the available aromatase inhibitors — anastrozole, letrozole and exemestane — in the adjuvant setting. Right now, there is only data with anastrozole. The other two agents are available for use by physicians, but there is no safety and efficacy data for them in the adjuvant setting. I have a reservation about saying that this is a class effect and in switching to another aromatase inhibitor for which we do not have any data.
| Selection of aromatase inhibitors as adjuvant therapy |
| Many reviewers have attempted to draw indirect comparisons between drugs within this class of aromatase inhibitors in an effort to identify the optimal aromatase inhibitor. . . .
This is fraught with difficulties, because randomized, controlled trials involving these agents have study designs with different criteria and different methods of assessment in different patient populations. As a result, such indirect comparisons between trials cannot possibly lead to a clear outcome in favor of any single drug. Some investigators even have attempted to reach conclusions based on the degree of estrogen suppression exhibited by aromatase inhibitors, but it should be noted that the net clinical relevance of plasma estrogen reduction still needs to be carefully evaluated. . . .
There are differences in both the chemistry and the pharmacological properties of the newer-generation aromatase inhibitors. These differences seem to have an impact upon selectivity of the drugs for aromatase (e.g., effect on adrenocorticotropic hormonestimulated cortisol levels) and may possibly have an effect on the clinical efficacy of aromatase inhibitors in the adjuvant setting on a long-term basis. |
| EXCERPT FROM: Buzdar A, Howell A. Clin Cancer Res 2001;7:2620-35. Abstract |
Clinical implications of the ATAC trial
The ATAC trial was designed to evaluate women who were just starting adjuvant hormonal therapy. In those women, we must discuss that we now have an agent — anastrozole — which appears to have a better safety and efficacy profile than tamoxifen. If I were the patient, I would go with the newer therapy — anastrozole — even though there is a shorter follow-up.
We would like all drugs to have follow-up for 20-30 years, but we cannot wait 20-30 years for the data to mature. We must provide women with the information and guide them as to the strengths and weaknesses of the data. The primary shortcoming of the ATAC trial data is its relatively short followup. On the other hand, the strengths of the data are the significant reduction in the risk of recurrence and the fewer side effects associated with anastrozole.
The ATAC trial does not address the woman who is free of recurrence and is already receiving adjuvant tamoxifen. Generally, a switch to an aromatase inhibitor should be considered only if a woman is experiencing substantial toxicity from tamoxifen. I would not — across the board — switch women who are tolerating tamoxifen. There are ongoing studies evaluating whether an aromatase inhibitor can further reduce the risk of recurrence in women who have received two or more years of adjuvant tamoxifen.
Implications of the ATAC results to breast cancer prevention trials
The dramatic reduction in second breast cancers observed with anastrozole in the ATAC trial has tremendous implications for breast cancer prevention. The Europeans are already planning to compare the efficacy of anastrozole and tamoxifen in high-risk women and in women with DCIS. We need to explore this further with definitive studies, particularly because postmenopausal women are at increased risk for thrombosis and endometrial cancer with tamoxifen.
The ATAC trial results clearly demonstrate that anastrozole has a much better safety profile than tamoxifen. The main safety concerns associated with tamoxifen — thromboembolic complications, vaginal bleeding, vaginal discharge and endometrial cancer — are related to its agonistic properties. A woman experiencing vaginal bleeding has to undergo a number of tests and procedures before we can rule out endometrial cancer. In the preliminary analysis of the ATAC trial results, vaginal bleeding was almost nonexistent with anastrozole; therefore, it is an attractive agent to evaluate for breast cancer prevention.
Adjuvant and neoadjuvant capecitabine/docetaxel (XT) trial
In the metastatic setting, capecitabine/docetaxel (XT) has demonstrated both survival and response rate advantages. Therefore, we plan to compare the XT combination and a taxane in terms of the ability to reduce the risk ofrecurrence in the adjuvant and neoadjuvant setting.
Patients will be randomized to either weekly paclitaxel for 12 weeks or three cycles of XT. Both regimens will be followed by four cycles of FAC. We chose weekly paclitaxel as our control arm, because of our prospective trial demonstrating that weekly neoadjuvant paclitaxel resulted in twice as many pathological complete responses as every-three-week paclitaxel. There is less data indicating that docetaxel is schedule-dependent.
We are going to use 75 mg/m2 of docetaxel and 1250 mg/m2 of capecitabine administered orally twice daily (morning and evening; equivalent to 2500 mg/m2 total daily dose) for two weeks followed by a one-week rest period given as three-week cycles.
We hope the toxicity will be manageable through capecitabine dose reductions. We have appropriate dose modification criteria for nonhematologic toxicity. If there is a neutropenic fever, we plan to add growth factors.
Women with an intact breast primary will receive a taxane as part of their therapy up front. We will determine if more women in the XT arm have a pathological complete response and breast preservation. Our previous experience indicates that women with pathological complete responses do very well in the long run. Therefore, a study arm with a higher pathological complete response rate will have fewer recurrences and disease-related morbidities or deaths.
Since XT has a higher likelihood of causing objective regression, I think more women receiving the combination will have a pathological complete response. There are a number of secondary end points in the study. In women with an intact primary, we will have the initial tumor and, subsequently, we can study what happens at the cellular or molecular level.
Experimental data indicate that docetaxel upregulates thymidine phosphorylase. To correlate this with outcome and response, we will be measuring these types of tumor factors at baseline and after therapy.
NSABP B-27: Neoadjuvant AC versus AC –> docetaxel
This trial clearly demonstrated that neoadjuvant, alternating, noncrossresistant chemotherapy regimens reduce the breast cancer volume. Women receiving docetaxel had almost twice as many pathological complete responses and more breast preservation than those receiving only four cycles of AC. I believe that once the data matures, more women receiving the docetaxel/AC combination will be alive and free of disease than those receiving AC alone.
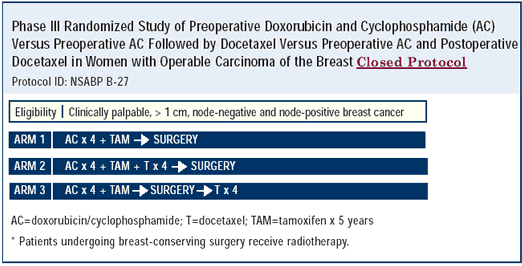
| PRELIMINARY RESULTS OF NSABP B-27: PREOPERATIVE AC/DOCETAXEL THE ADDITION OF DOCETAXEL TO AC ON PRIMARY TUMOR RESPONSE RESULTED IN: |
• Equivalent rates of breast-conserving surgery and mastectomy
• Significantly increased clinical (65% to 40%) and pathological (25.6% to 13.7%) complete response rates
• A higher percentage of patients with histological negative axillary nodes (59.5% to 51.5%)
• Additional grade 4 toxicity (24% to 10%) Note: 2,411 patients randomized, with an average time on study of approximately 40 months |
| Derived from NSABP Presentation, 2001 San Antonio Breast Cancer Symposium. Abstract 5. |
Neoadjuvant trastuzumab trial
In women with HER2-positive tumors — either 3+ on HercepTest™ or FISHpositive — we are conducting a single-institution neoadjuvant trastuzumab trial. All women receive four cycles of paclitaxel and four cycles of FEC with or without concurrent, weekly trastuzumab. We are employing very close cardiac monitoring. We thought epirubicin offered a lower risk of cardiac toxicity, and it was specifically chosen for that reason. Since we are only giving four cycles of FEC, the total cumulative dose of epirubicin and, hence, the risk of cardiac dysfunction should be very low.
Select publications
|
