| You are here: Home: BCU 4|2002: Kent Osbourne, MD
 |
 |
 |
 |
 |
C Kent Osborne, MD |
 |
 |
Professor, Departments of Medicine and
Molecular & Cellular Biology
Director, Baylor Breast Center
Baylor College of Medicine
Director, Baylor Breast Cancer Specialized
Program of Research Excellence Grant |
|
 |
 |
 |
Edited comments by Dr Osborne
Results of the ATAC trial: Anastrozole versus tamoxifen versus anastrozole/tamoxifen
This study establishes a new paradigm in blocking the estrogen receptor pathway. It demonstrates that decreasing the estrogen concentration to very low levels is a more potent way to attack the receptor than is tamoxifen. It is logical that the combination arm in the trial was inferior to the anastrozole arm. Tamoxifen has intrinsic estrogenic activity. Therefore, combining it with an agent that lowers estrogen levels — anastrozole — is counterproductive and gives the same result as with tamoxifen alone.
Clinical applicability of the ATAC trial
I discuss anastrozole with my ER-positive postmenopausal patients. Anastrozole was better than tamoxifen with regard to endometrial cancer, thromboembolic events and hot flashes. Tamoxifen was better with regard to bone fractures. Baseline bone densities will likely be indicated, and I suspect that some women on aromatase inhibitors for long periods of time will need treatment with bisphosphonates. My higher-risk patients will generally receive an aromatase inhibitor, while the lower-risk patients tend to receive tamoxifen — primarily because of the bone issue. I also use aromatase inhibitors in women with a propensity for or history of thrombosis. I believe that as we obtain more data, the aromatase inhibitors will be used more frequently. I talk with patients about anastrozole, because that’s the aromatase inhibitor for which we have data.
Potential advantages of bisphosphonates in breast cancer patients
Bisphosphonates will help preserve bone density and reduce the chance of osteoporosis. They may also help as a breast cancer therapy by making the bones less “fertile soil” for breast cancer cells. Bisphosphonates turn off osteoclast-induced resorption of bone, reduce growth factors and cause apoptosis of tumor cells. These agents may actually be an indirect antitumor therapy.
It will be interesting to see the results of some of the ongoing adjuvant trials using the bisphosphonates, pamidronate and clodronate. Although there is still some inconsistency in the trial results, bisphosphonates likely will be useful agents.
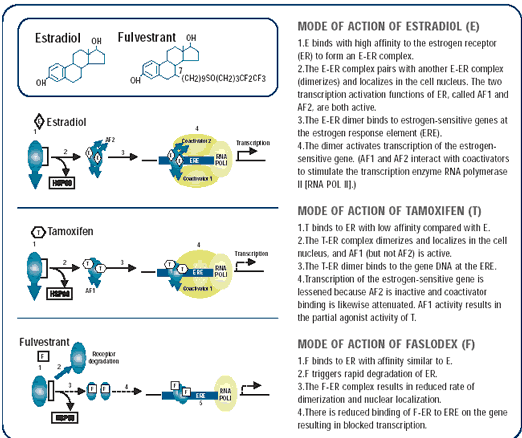
Mechanisms of action of fulvestrant: An estrogen receptor downregulator
If you look at the spectrum of drugs that interact with the estrogen receptor, estrogen is on one end of the spectrum, stimulating most genes under its control after binding to the estrogen receptor. Drugs like tamoxifen stimulate some genes and inhibit others, depending on the tissue and gene. At the far other end of the spectrum, there are drugs like fulvestrant that seem to have a predominantly pure antiestrogenic profile on all genes with none of the agonist qualities of tamoxifen.
There are several activation domains on the estrogen receptor protein — areas that seem to be important in activating transcription of genes.
Tamoxifen only blocks one of these — probably the most important one — but it only blocks one. The other one is still active, and this may give rise to the agonist qualities of tamoxifen.
In contrast, fulvestrant blocks all of the activation domains on the receptor, including both AF-1 and AF-2. Fulvestrant also reduces the level of the estrogen receptor in cells. In some cases, you can’t measure any estrogen receptor after exposure to fulvestrant. So, fulvestrant doesn’t have any agonist activity, blocks all transcription domains and eliminates the estrogen receptor.
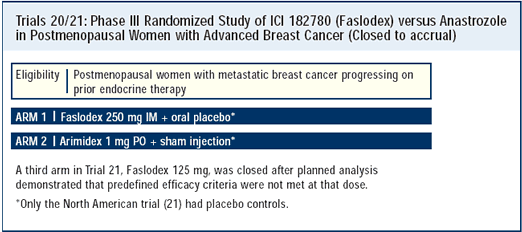
Second-line trials for metastatic disease: Fulvestrant versus anastrozole
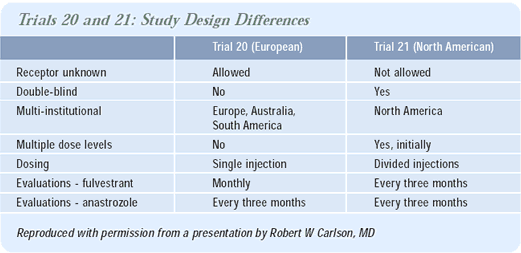
The European trial and the American trial are a bit different in their structure and results. The American trial — which I think has a better design — was a double-blind study. Patients assigned to anastrozole received placebo injections. Also, because the patients in both groups had to visit the clinic once a month, there was consistency with regard to patient evaluations.
The European trial was not double-blinded. The patients on anastrozole were seen every three months, while the patients on fulvestrant were seen every month. This design has the potential to have some bias in terms of identifying when the patients progressed. Patients in the fulvestrant group of the European trial were seen more often, and conceivably progression would be identified a little earlier than in a patient randomized to anastrozole.
The results show similar response rates between the two drugs. However, in the American trial, the response duration is about twice as long for fulvestrant compared to anastrozole. We have to acknowledge that aromatase inhibitors are very good agents in and of themselves, and in one of these new trials, fulvestrant is at least as good as anastrozole. In the other trial we see an advantage at least in one important parameter.
Tolerability of fulvestrant
This agent is very well tolerated. In clinical trials, the monthly injection didn’t cause much pain or discomfort. Both anastrozole and fulvestrant were very well tolerated in the randomized clinical trial. There are theoretical reasons why fulvestrant might not cause hot flashes because it doesn’t seem to cross the blood-brain barrier. In these studies, the rate of hot flashes and other side effects with both fulvestrant and anastrozole was very low.
We know very little about fulvestrant’s effect on bone and lipids. These pure antiestrogens could theoretically be deleterious, which is not as important for metastatic breast cancer as it will be if this agent moves into adjuvant therapy and prevention. Additional studies are needed to clarify these issues, some of which can be dealt with by using other therapies. For example, in patients with low bone density, bisphosphonates could theoretically be used. In the end, if it is a much better cancer drug, these concerns will be secondary.
Fulvestrant in the adjuvant setting
Adjuvant trials of fulvestrant are in the planning stage. If the data that become available continue to look promising, I believe fulvestrant will move forward into the adjuvant situation. In almost every cancer therapy, we see greater benefits in the adjuvant setting than in the metastatic setting. We are seeing this with anastrozole now — it doesn’t cure metastatic disease, but in the adjuvant situation with micrometastatic disease, the effects are much greater. The effects of fulvestrant that we might see in the adjuvant situation are likely to be much greater than in the metastatic setting.
Combining fulvestrant and anastrozole
It would be intriguing to combine fulvestrant with an aromatase inhibitor. This would reduce the ligand, estradiol, to a very low level, and it would deplete the estrogen receptor. I believe a large randomized trial — comparing an aromatase inhibitor versus fulvestrant versus the combination — will be done.
The combination arm of the ATAC trial yielded a worse outcome than anastrozole alone. This makes some biological sense because of tamoxifen’s partial estrogen agonist activity. However, when fulvestrant binds the receptor, there is a different outcome than with tamoxifen. When we examine the differences in these drugs at the molecular level, it is logical that the combination of fulvestrant and anastrozole may be effective.
Biology of HER2-positive, ER-positive tumors
The estrogen receptor pathway is not as simple as we once thought. An important component to estrogen’s growth-stimulating effects on tumors that have amplification of the HER2 oncogene involves growth factor pathways. Growth factors can phosphorylate and activate the estrogen receptor and the estrogen receptor coactivator AIB-1. When these are activated, SERMs like tamoxifen are converted into potent estrogens with very little antagonist activity.
Our laboratory studied HER2 and AIB-1 levels in human tumors. The data show that tumors with high levels of AIB-1, estrogen receptor and HER2 don’t benefit from tamoxifen. This clinical data provides a rationale to inhibit these growth factor pathways. There is evidence that blocking the growth factor receptor with agents such as Iressa® (ZD 1839) or trastuzumab prevents tamoxifen-stimulated growth and restores tamoxifen’s antagonistic activity.
HER2-positive, ER-positive patients generally don’t benefit from endocrine therapy as much as HER2-negative, ER-positive women. However, we’re only measuring one component with HER2 status. In our study, only tumors with high levels of both AIB-1 and HER2 lack response to tamoxifen. Patients with high HER2 levels but low AIB-1, or high AIB-1 but low HER2, benefit from tamoxifen. Approximately 60% of tumors that overexpress HER2 also overexpress AIB-1. Therefore, 10 to 15 percent of tumors express both. In my own practice, I give ER-positive, HER2-positive patients endocrine therapy, knowing that some patients won’t benefit.
Treating the ER-positive, HER2-positive patient
In the past, I used tamoxifen in these women, but now I tend to use aromatase inhibitors. Data from my own laboratory show that HER2 overexpressing tumors respond much better to estrogen deprivation than to tamoxifen. Using an aromatase inhibitor before tamoxifen makes a lot of sense in that situation.
If the patient progresses after endocrine therapy, my treatment depends on the situation. I would be inclined to try a receptor tyrosine kinase inhibitor like trastuzumab alone in patients with indolent disease and then add chemotherapy if they don’t respond or at the time of progression. This is a way to keep a good quality of life. If the patient has a lot of visceral disease, I might employ a taxane or another drug that may have an additive or synergistic effect with trastuzumab. This would depend on the patient’s prior adjuvant chemotherapy and time to progression after completion of adjuvant chemotherapy.
Duration of trastuzumab therapy
When my patients progress on single-agent trastuzumab or trastuzumab with chemotherapy, I usually continue trastuzumab and add another chemotherapeutic agent. I don’t know if this is the right thing to do — we need to study that further. There is some data to suggest that trastuzumab alone does not inhibit the tumor but rather helps the chemotherapy work better. So, I tend to continue trastuzumab as long as we’ve elected to use chemotherapy.
Select publications
|
