| You are here: Home: BCU 4|2002: Nicholas J Robert, MD
 |
 |
 |
 |
 |
Nicholas J Robert, MD |
 |
 |
Inova Fairfax Hospital
Chairman, US Oncology Breast Cancer Research Committee
Chairman of Research, Inova Fairfax Hospital
Member, Eastern Cooperative Oncology Group (ECOG)
Member, National Surgical Adjuvant Breast and Bowel Project (NSABP) |
|
 |
 |
 |
Edited comments by Dr Robert
Phase II trial of trastuzumab in combination with carboplatin and paclitaxel for metastatic breast cancer
The pivotal trial by Slamon demonstrated the utility of using chemotherapy with trastuzumab. There was significant cardiotoxicity in those patients receiving AC plus trastuzumab, so that was not a regimen that could be developed further.
We designed a phase II trial that attempts to improve upon the trastuzumab/paclitaxel combination by adding in carboplatin. There is synergy between the platinum salts and trastuzumab, and clinical data from Edith Perez and the North Central Cancer Therapy Group demonstrated a 60% response rate from every-three-week paclitaxel and carboplatin. David Loesch from US Oncology obtained similar results with the same combination given on a different schedule.
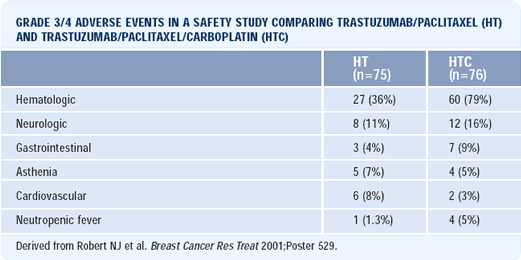
Our study compares paclitaxel/trastuzumab to the combination of paclitaxel, trastuzumab and carboplatin. The target accrual is 205 patients, which is nearly completed. In terms of safety, preliminary data suggests that the two arms are comparable, with the exception of increased myelosuppression from adding in carboplatin.
Cardiotoxicity was not problematic, except for patients with a prior history of cardiac disease. We did not detect declines in left ventricular ejection fraction — relative to baseline — even in patients who received adjuvant anthracyclines.
Pilot studies of trastuzumab with a taxane/platinum combination
Two pilot studies from the BCIRG and UCLA evaluated trastuzumab with a taxane/platinum regimen in HER2 FISH-positive patients with metastatic breast cancer. Improved response rates were demonstrated in the study utilizing carboplatin but not for cisplatin. Howard Burris at the Sarah Cannon Cancer Center gave trastuzumab up front to IHC 2/3+ HER2- positive patients with metastatic breast cancer. After eight weeks, patients with stable disease or progression were treated with paclitaxel and carboplatin with or without trastuzumab, and significant responses were seen.
| Trastuzumab in combination with platinum agents |
| The combination of trastuzumab with platinum analogs was one of the initial strategies tested in the early development of trastuzumab. Although these studies demonstrated that the combination was well tolerated and showed antitumor efficacy, this strategy was not pursued further because cisplatin and carboplatin are not generally used in the treatment of breast cancer. Preclinical studies have demonstrated significant synergy between trastuzumab and both carboplatin and carboplatin plus docetaxel or paclitaxel. In addition, further studies have elucidated the probable mechanism for this synergy, which involves inhibition by trastuzumab of the repair of the DNA adducts formed due to cisplatin therapy. |
| EXCERPT FROM: Winer EP, Burstein HJ. Oncology 2001;61(suppl):50-57. Abstract |
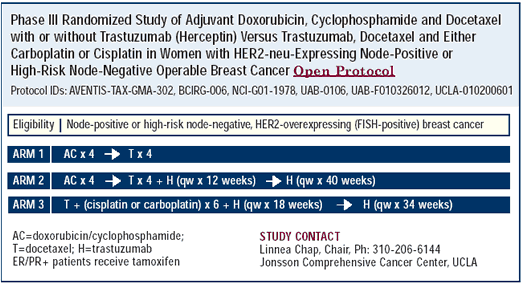
BCIRG 006 adjuvant trial
The pilot studies of trastuzumab combined with a taxane/platinum regimen were the impetus for the large adjuvant trial being conducted by the Breast Cancer International Research Group. It is a very well-designed study, which evaluates three regimens in HER2-positive, node-positive and high-risk nodenegative women with primary breast cancer. In two arms, patients receive doxorubicin and cyclophosphamide followed by docetaxel with or without trastuzumab. The third arm represents a departure from the standard approach of using anthracyclines in the adjuvant setting; patients will receive docetaxel, a platinum salt — carboplatin or cisplatin — and trastuzumab.
First-line therapy for HER2-positive metastatic breast cancer
I use the combination of paclitaxel and trastuzumab in HER2-positive patients with metastatic disease, but I am eagerly awaiting the results of our trial to determine if carboplatin adds to this regimen. Pilot studies conducted by Nabholtz and Slamon, which added carboplatin to trastuzumab/taxane, demonstrated prolongation in time to progression — 12 months in one trial and 17 months in the other. These compare very favorably to the seven months in time to progression seen in the trastuzumab pivotal trial. Three-drug combinations may turn out to be the preferred strategy for HER2-positive patients.
Trastuzumab monotherapy is also an attractive therapeutic approach. It is analogous to the use of sequential single-agent endocrine therapy for indolent metastatic disease. HER2-positive tumors are not necessarily always aggressive. Chuck Vogel demonstrated a very acceptable response rate and clinical benefit with single-agent trastuzumab. Howard Burris and his colleagues gave trastuzumab up front and used chemotherapy in those who failed to respond or progressed. This is a reasonable strategy and should be considered in appropriately chosen patients.
Duration of trastuzumab use
I continue trastuzumab as long as the patient has no evidence of cardiac problems. This strategy is unproven, but it is being evaluated in a trial conducted by MD Anderson. In this study, patients will be randomized to second-line therapy with vinorelbine or trastuzumab and vinorelbine. Typically, I use a trastuzumab/taxane combination for six months, then switch to singleagent trastuzumab. In the pivotal trial, most patients did not stay on chemotherapy indefinitely. If a patient fails trastuzumab and paclitaxel, then I will continue with single-agent trastuzumab and add in another synergistic agent, like vinorelbine.
Trastuzumab use after ACT adjuvant therapy
Women with HER2 overexpressing tumors who have failed prior ACT adjuvant therapy are a challenge to manage, and I consider when they failed that regimen and how they received the taxane. If they received a taxane on an every-three-week schedule, a weekly taxane can be used as salvage therapy, and I would be comfortable using trastuzumab with a taxane in that situation.
Clinical implications of the ATAC trial results
These data will have a huge impact on the clinical care of our patients. Diseasefree survival and the toxicity profile were more favorable for anastrozole. There was less weight gain and fewer hot flashes with anastrozole but more arthralgias and concerns about bone density. This is definitely an option that we will need to discuss with our patients. If patients ask for advice, I will recommend anastrozole.
Switching patients from tamoxifen to anastrozole
For a woman who has received tamoxifen for two to three years, it is reasonable to continue that therapy. However, the ATAC trial results should be communicated to patients, and some will choose to switch to anastrozole. I would be comfortable with that decision. One cannot evaluate the ATAC trial in isolation. There are multiple trials that have demonstrated that aromatase inhibitors are superior to tamoxifen. The first lead in the adjuvant setting is a disease-free survival advantage; survival advantages are rarely seen in earlier analyses. At this time, the use of adjuvant anastrozole is analogous to the switch made in the late 1980s of offering chemotherapy to high-risk, node-negative patients when an advantage was demonstrated in disease-free survival. As the data were unfolding, some argued that it was necessary to see a survival advantage, but I believe that would have deprived patients of a successful intervention. Making decisions about when to adopt a new therapy is really the art of medicine interacting with science.
Interchangeability of aromatase inhibitors
Anastrozole was superior to tamoxifen in the ATAC trial. There are no data comparing the other aromatase inhibitors to tamoxifen in the adjuvant setting. Eventually letrozole and exemestane may also be shown to be better than tamoxifen as adjuvant therapy, but we do not have that data, so I would use anastrozole.
Adjuvant endocrine maneuvers in ER-positive premenopausal patients
In high-risk, ER-positive premenopausal women who continue to menstruate after chemotherapy, it is reasonable to use ovarian ablation/suppression. Nancy Davidson's update of the Intergroup 0101 trial demonstrated an advantage for adding goserelin and tamoxifen to CAF chemotherapy. Another strategy would be ovarian ablation/suppression plus an aromatase inhibitor. I have had excellent results with this combination in a few patients with metastatic disease, who continued menstruating and did not want chemotherapy. Interestingly, combination endocrine maneuvers have generally been unsuccessful. The newer aromatase inhibitors and fulvestrant have renewed interest in the idea that these agents may be additive with other endocrine interventions.
Phase III trial of fulvestrant versus anastrozole as second-line therapy in ER-positive postmenopausal patients
The North American trial suggested that fulvestrant may be superior to anastrozole as second-line therapy for metastatic disease, but overall, fulvestrant is at least comparable to anastrozole. We are fortunate to now have another useful endocrine intervention for our patients and another option to control their disease using a nonchemotherapy intervention. A higher dosage of fulvestrant may be even more effective, and this should be addressed in a clinical trial. The monthly intramuscular injection has not been problematic, particularly compared to chemotherapy. Fulvestrant is well tolerated, and I expect that patients will receive it favorably.
Select publications
|
