 |
![]()
Select Excerpts from the Discussion
Track 38
![]() DR LOVE: Joe, can you discuss the ASCO Guidelines Committee’s
recommendations, published in the November 2007 Journal of Clinical
Oncology, about the use of tumor markers in breast cancer?
DR LOVE: Joe, can you discuss the ASCO Guidelines Committee’s
recommendations, published in the November 2007 Journal of Clinical
Oncology, about the use of tumor markers in breast cancer?
![]() DR SPARANO: The panel found sufficient evidence to recommend multi-parameter
gene expression analysis. They also found sufficient evidence to
recommend measuring urokinase plasminogen activator (uPA) or plasminogen
activator inhibitor 1 (PAI-1) using ELISA, which requires a minimum of 300
milligrams of fresh or frozen tissue. Low levels of uPA/PAI-1 are associated
with a good prognosis with tamoxifen alone for patients with ER-positive
disease (Harris 2007), although issues exist regarding the effect of core biopsies
on the results.
DR SPARANO: The panel found sufficient evidence to recommend multi-parameter
gene expression analysis. They also found sufficient evidence to
recommend measuring urokinase plasminogen activator (uPA) or plasminogen
activator inhibitor 1 (PAI-1) using ELISA, which requires a minimum of 300
milligrams of fresh or frozen tissue. Low levels of uPA/PAI-1 are associated
with a good prognosis with tamoxifen alone for patients with ER-positive
disease (Harris 2007), although issues exist regarding the effect of core biopsies
on the results.
![]() DR LOVE: What are uPA and PAI-1?
DR LOVE: What are uPA and PAI-1?
![]() DR SPARANO: They are an index of the fibrinolytic system and biological
processes involved in metastasis. In terms of other markers, the panel found
insufficient evidence for the use of IHC-based markers as they relate to
measuring proliferation, including Ki-67, cyclin D, cyclin E, p27, p21, thymidine
kinase and topoisomerase II (Harris 2007).
DR SPARANO: They are an index of the fibrinolytic system and biological
processes involved in metastasis. In terms of other markers, the panel found
insufficient evidence for the use of IHC-based markers as they relate to
measuring proliferation, including Ki-67, cyclin D, cyclin E, p27, p21, thymidine
kinase and topoisomerase II (Harris 2007).
They also found insufficient evidence for the use of other markers that have been recently published, including cyclin E fragments, proteomic analysis and circulating tumor cells.
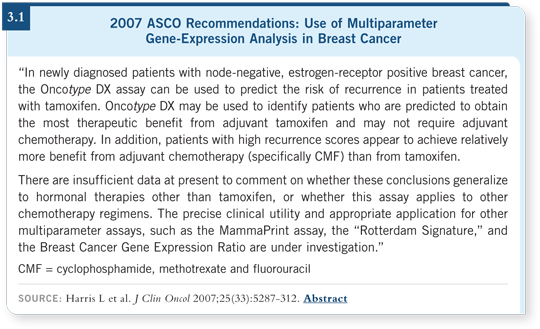
Regarding multiparameter gene-expression analysis, the ASCO panel concluded that the Oncotype DX assay can be used to predict the risk of recurrence in newly diagnosed patients with node-negative, ER-positive breast cancer who are treated with tamoxifen. In addition, it may be used to identify patients who are predicted to attain the most therapeutic benefit from adjuvant tamoxifen and may not require chemotherapy. Conversely, they concluded that patients with tumors with a high Recurrence Score achieve more benefit from adjuvant chemotherapy, specifically CMF, than from tamoxifen alone (Harris 2007; [3.1]).
The MammaPrint® assay appears to identify groups of patients with a particularly good or particularly poor prognosis. However, considering the design of studies used to validate the assay, it is uncertain whether the data pertain to an inherently favorable outcome in untreated patients, to patients whose prognosis is favorable due to therapy or to those with poor outcomes in the absence of treatment or despite treatment.
In addition, the need for fresh or frozen tissue makes this assay challenging in current clinical practice. The panel concluded that more definitive recommendations for the use of the MammaPrint assay in clinical practice will require data from more clearly directed studies (Harris 2007).
Track 40
![]() DR LOVE: Do we have any data correlating the MammaPrint assay with
benefit from chemotherapy?
DR LOVE: Do we have any data correlating the MammaPrint assay with
benefit from chemotherapy?
![]() DR SPARANO: I believe that’s one of the critical and key differences between
how the MammaPrint and the Oncotype DX assays were developed and
validated. They may be equally fine in terms of predicting outcomes, but they
were developed in different ways.
DR SPARANO: I believe that’s one of the critical and key differences between
how the MammaPrint and the Oncotype DX assays were developed and
validated. They may be equally fine in terms of predicting outcomes, but they
were developed in different ways.
The Oncotype DX assay was developed using data from prospective randomized trials comparing tamoxifen to placebo (Paik 2004) or tamoxifen to tamoxifen with CMF (Paik 2006), which is different from how the groups of patients were studied with the MammaPrint assay (van ‘t Veer 2002; van de Vijver 2002).
A second key difference is how the tissue is processed. For the MammaPrint assay, or any assay that requires collection of fresh tissue, you have to know in advance that the patient is a potential candidate for the test. A third important issue is the transparency of Oncotype DX as opposed to MammaPrint. MammaPrint involves a Pandora’s box of 70 genes that we know nothing about. In contrast, Oncotype DX involves a much more manageable number of genes that are familiar to us as clinicians — they make sense.
Track 41
![]() DR LOVE: Joe, can you discuss the potential role of Oncotype DX for
patients with node-positive disease?
DR LOVE: Joe, can you discuss the potential role of Oncotype DX for
patients with node-positive disease?
![]() DR SPARANO: At the 2004 San Antonio Breast Cancer Symposium, Kathy
Albain presented the results from SWOG-8814, evaluating five years of
tamoxifen alone or in combination with CAF for postmenopausal patients
with node-positive, ER-positive disease. A better outcome was demonstrated
for those who received CAF followed by sequential tamoxifen compared to
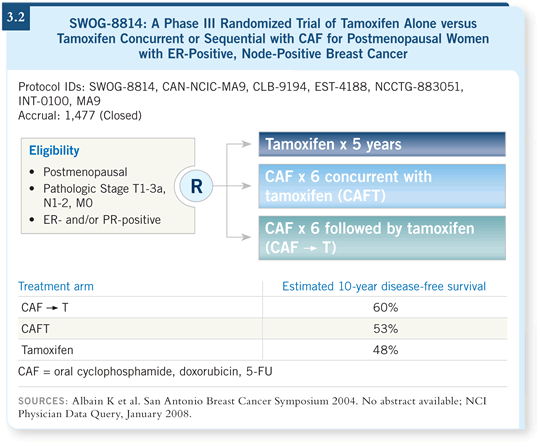
those who received tamoxifen alone (Albain 2004; [3.2]).
DR SPARANO: At the 2004 San Antonio Breast Cancer Symposium, Kathy
Albain presented the results from SWOG-8814, evaluating five years of
tamoxifen alone or in combination with CAF for postmenopausal patients
with node-positive, ER-positive disease. A better outcome was demonstrated
for those who received CAF followed by sequential tamoxifen compared to
those who received tamoxifen alone (Albain 2004; [3.2]).
More recently, she evaluated whether the Oncotype DX assay provided information additional to the clinical features in a subset of about 40 percent of the patients in the parent trial (Albain 2007).
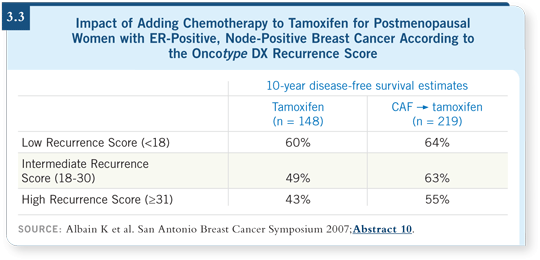
In SWOG-8814, patients with a Recurrence Score of 31 or higher obtained a significant reduction in the risk of recurrence with the addition of chemotherapy. Those who had a low Recurrence Score obtained no benefit.
Among those with intermediate Recurrence Scores, a trend appeared toward benefit from the addition of chemotherapy, but the p-value was not statistically significant (Albain 2007; [3.3]). Examining these data with Forest plots reveals a strong trend favoring the administration of chemotherapy to patients who have an intermediate Recurrence Score (Albain 2007).
TOPICS
Challenges in HER2 Testing and Interpretation
- Select publications
Assessment of Estrogen Receptor Status
- Select publications
Evolving Role of Genomic Assays in Breast Cancer
- Select publications
Tissue Biomarkers in the Management of Breast Cancer:
A CME Audio Series and Activity