 |
![]()
Select Excerpts from the Discussion
Tracks 20-22
![]() DR LOVE: Craig, can you discuss the study by Kim on ER testing?
DR LOVE: Craig, can you discuss the study by Kim on ER testing?
![]() DR ALLRED: Kim and colleagues compared three assays for the measurement
of ER status in patients with node-negative, ER-positive breast cancer. The
three assays were a ligand-binding assay, IHC and quantitative RT-PCR. This
study was based on a subset of 297 patients treated with tamoxifen in NSABPB-14, one of the pivotal trials demonstrating the efficacy of adjuvant tamoxifen
in patients with node-negative, ER-positive breast cancer (Kim 2006).
DR ALLRED: Kim and colleagues compared three assays for the measurement
of ER status in patients with node-negative, ER-positive breast cancer. The
three assays were a ligand-binding assay, IHC and quantitative RT-PCR. This
study was based on a subset of 297 patients treated with tamoxifen in NSABPB-14, one of the pivotal trials demonstrating the efficacy of adjuvant tamoxifen
in patients with node-negative, ER-positive breast cancer (Kim 2006).
The ligand-binding assay, performed at the study sites, required fresh or frozen tissue. IHC was performed on formalin-fixed tissue sections in a central laboratory at NSABP with an FDA-approved, validated kit — the DakoCytomation ER/PR pharmDx™. They used computerized-image analysis to quantify results three ways: percent of positive cells, average intensity of positive cells and the product of both. Quantitative RT-PCR required formalin-fixed tissue and was performed at Genomic Health as part of the Oncotype DX 21-gene panel. ER is one of those genes (Kim 2006).
The aim of the study was to determine the level of correlation between the different assays and the relationship of each assay to the clinical outcome of distant recurrence-free interval. The correlation between the assays varied, and the correlations between all of the assays and the overall Oncotype DX Recurrence Score were weak (Kim 2006).
This retrospective study demonstrated a notable difference in the ability of these tests to predict the distant recurrence-free interval with tamoxifen. The ligand-binding assay performed most poorly, with a hazard ratio of 0.86 that was not statistically significant. The different methods for scoring the IHC assay demonstrated hazard ratios ranging from about 0.3 to 0.6, and they were all statistically significant in univariate analysis. RT-PCR was remarkably predictive in this single study. The hazard ratio was 0.14 and highly statistically significant (Kim 2006; [2.1]).
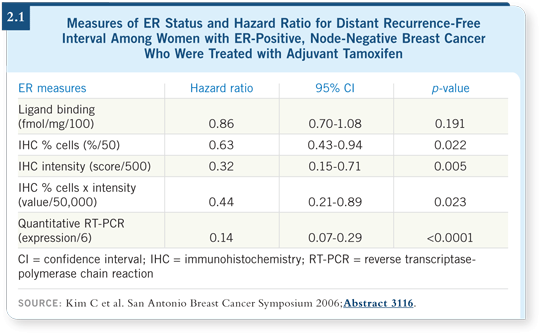
These are promising preliminary results, suggesting that evaluating ER by quantitative RT-PCR is more predictive — compared to IHC or the ligand-binding assay — of response to tamoxifen in patients with ER-positive disease. However, this is only one study.
Quantitative RT-PCR performed almost too well: Hazard ratios as low as 0.14 are almost unbelievable. We’ve never seen anything like that before. It’s wonderful if it’s true and reproducible, but we don’t know that yet.
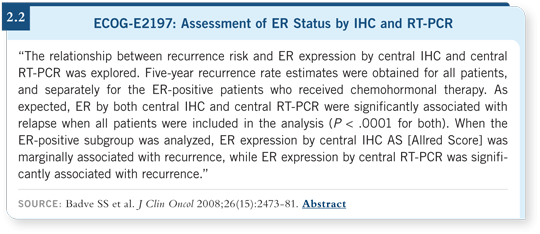
Recently some of the same investigators published a similar study evaluating ER status assessed by IHC versus RT-PCR in ECOG-E2197, a trial for patients with high-risk breast cancer who received chemotherapy with or without tamoxifen based on hormone receptor status (Badve 2008; [2.2]). RT-PCR was a significant predictor of recurrence, but it didn’t have a hazard ratio anywhere near 0.14 that was seen in the Kim paper (Kim 2006; [2.1]).
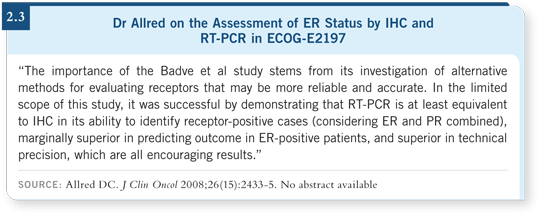
I wrote an editorial about that paper (Allred 2008; [2.3]). The performance of IHC and RT-PCR were similar. We saw a trivial statistical advantage to RT-PCR, but they both did well. So that raises the question, does this just happen to be a sampling bias in this particular retrospective study? I don’t know. I believe the results, but these aren’t the same results as those from another study conducted by some of the same people.
Track 24
![]() DR LOVE: The Oncotype DX assay now reports quantitative ER. Are
there situations in which that information might change what you do?
DR LOVE: The Oncotype DX assay now reports quantitative ER. Are
there situations in which that information might change what you do?
![]() DR BUDD: Not for me right now. The following decisions need to be made:
Do we or do we not use hormonal therapy? Do we or do we not use chemotherapy?
If the patient has ER-positive disease, we will use hormonal therapy.
Then the decision is whether to use chemotherapy. To make that decision,
you’re using the whole Oncotype DX Recurrence Score.
DR BUDD: Not for me right now. The following decisions need to be made:
Do we or do we not use hormonal therapy? Do we or do we not use chemotherapy?
If the patient has ER-positive disease, we will use hormonal therapy.
Then the decision is whether to use chemotherapy. To make that decision,
you’re using the whole Oncotype DX Recurrence Score.
![]() DR SPARANO: I believe at the least it provides greater transparency.
DR SPARANO: I believe at the least it provides greater transparency.
You struggle when you have a midrange Recurrence Score. If you had the information regarding ER and PR and they were high, as a clinician you’d feel more confident in recommending to that patient, “I believe you’ll be okay with endocrine therapy alone.”
I believe it will be more helpful for the patients who have intermediate Recurrence Scores because we already know from a great deal of experience what happens at the extremes. The proportion of patients with intermediate Recurrence Scores, no matter how you define it, can be anywhere from 45 to 70 percent, depending on how you select your patients.
![]() DR GOSS: I agree with Tom. The decision to use chemotherapy based on the
Oncotype DX assay is primarily driven by the Recurrence Score overall, and
the Recurrence Score has the ER level built into it.
DR GOSS: I agree with Tom. The decision to use chemotherapy based on the
Oncotype DX assay is primarily driven by the Recurrence Score overall, and
the Recurrence Score has the ER level built into it.
I don’t believe the decision to use chemotherapy for patients with intermediate Recurrence Scores should be influenced by the quantitative ER results, but you could argue that the choice of endocrine therapy or the type of endocrine therapy might be so influenced.
![]() DR SPARANO: I take the opposite view. We know that a continuous relationship
exists between the Recurrence Score and benefit from chemotherapy — the
higher the score, the greater the benefit. We don’t know at what threshold you
do not benefit from chemotherapy. So for the 40 to 70 percent of patients with
intermediate Recurrence Scores, I would have more confidence relying on
endocrine therapy alone if I had a higher level of ER and/or PR expression.
DR SPARANO: I take the opposite view. We know that a continuous relationship
exists between the Recurrence Score and benefit from chemotherapy — the
higher the score, the greater the benefit. We don’t know at what threshold you
do not benefit from chemotherapy. So for the 40 to 70 percent of patients with
intermediate Recurrence Scores, I would have more confidence relying on
endocrine therapy alone if I had a higher level of ER and/or PR expression.
Tracks 26-27
![]() DR LOVE: Craig, can you comment on the new analysis for the P024
study by Ellis evaluating neoadjuvant endocrine therapy?
DR LOVE: Craig, can you comment on the new analysis for the P024
study by Ellis evaluating neoadjuvant endocrine therapy?
![]() DR ALLRED: In P024, postmenopausal patients with Stage II/III, ER-positive
disease were randomly assigned to neoadjuvant tamoxifen or letrozole for four
months. Then the tumor was surgically removed, usually by lumpectomy,
and thereafter all patients received tamoxifen for up to five years. The median follow-up at the time of this analysis was about 62 months, slightly over five
years (Ellis 2008).
DR ALLRED: In P024, postmenopausal patients with Stage II/III, ER-positive
disease were randomly assigned to neoadjuvant tamoxifen or letrozole for four
months. Then the tumor was surgically removed, usually by lumpectomy,
and thereafter all patients received tamoxifen for up to five years. The median follow-up at the time of this analysis was about 62 months, slightly over five
years (Ellis 2008).
Tissues were sampled twice for proliferation rate, hormone receptor status, grade, tumor size and nodal status, first at the initial diagnosis by core biopsy and then from the excised tumor after four months of neoadjuvant hormonal therapy. The analysis included looking for univariate correlations, developing a multivariate model and performing internal validation on all of these parameters, which are standard in a neoadjuvant setting (Ellis 2008).
Rather than conducting this core biopsy analysis on the primary tumor, it was done on the tumor after four months of therapy. So theoretically you have a chance to measure the response of the tumor under the pressure of therapy. The variables all correlated with both relapse-free and breast cancer-specific survival. In the new update, the investigators have also had an opportunity to validate their prognostic model in an independent data set (Ellis 2008).
On multivariate analysis, only four parameters — tumor size, node status, Ki-67 proliferation rate and ER status — remained significant for relapse-free survival. When the endpoint was changed to breast cancer-specific survival, the same four variables remained significant on multivariate analysis. The investigators remodeled only those four variables to recalculate the hazard ratios, which were strengthened and remained significant (Ellis 2008).
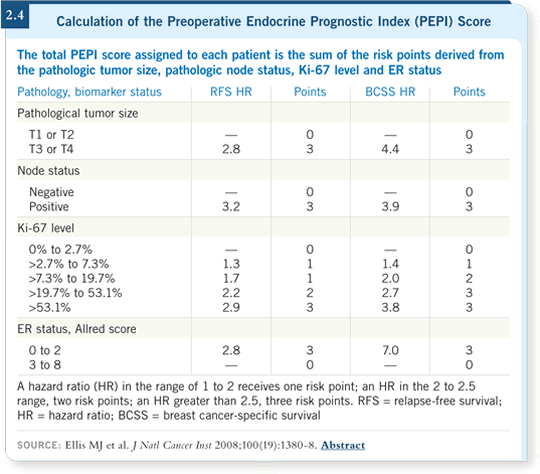
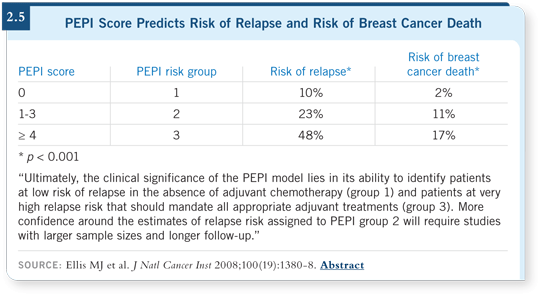
Then they used these hazard ratio data to create what they referred to as the preoperative endocrine prognostic index (PEPI). They followed a strategy from the cardiovascular literature that developed a numerical score for predicting outcomes for patients who experienced myocardial infarctions.
They measured multiple variables and assigned points based on the magnitude of the hazard ratios. If a hazard ratio was between one and two, it was assigned one point. If it was between two and 2.5, it had two points, and so on (Ellis 2008; [2.4]).
The PEPI score showed remarkable ability to predict response to therapy. At five years, recurrence-free survival among patients in the lowest-risk tertile (PEPI risk group 1) was almost 95 percent. This led the authors to conclude that these patients could be treated without adjuvant chemotherapy. Survival was much worse in the PEPI risk groups 2 and 3 (Ellis 2008; [2.5]).
For independent validation, investigators applied the PEPI model to patients in the IMPACT trial, which evaluated treatment for three months with anastrozole, tamoxifen or the combination before surgery. Relapse-free survival was 100 percent in the PEPI risk group 1, with much poorer results in the higher PEPI risk groups (Ellis 2008).
The authors concluded, and I agree with most of their conclusions, that the PEPI score is a powerful and inexpensive tool for predicting relapse in postmenopausal women with Stage II/Stage III ER-positive breast cancer.
After neoadjuvant hormonal therapy, patients with a PEPI score of zero, which accounts for approximately 10 percent of the patient population, can probably be treated without adjuvant chemotherapy and remain on hormonal therapy alone. This would be a major change in therapeutic strategy.
TOPICS
Challenges in HER2 Testing and Interpretation
- Select publications
Assessment of Estrogen Receptor Status
- Select publications
Evolving Role of Genomic Assays in Breast Cancer
- Select publications
Tissue Biomarkers in the Management of Breast Cancer:
A CME Audio Series and Activity