 |
![]()
Select Excerpts from the Discussion
Tracks 3-4
![]() DR LOVE: Antonio, you were the lead author for the paper about HER2
testing that came out of an elite group from ASCO and CAP (Wolff
2007a, 2007b). Would you discuss the conclusions?
DR LOVE: Antonio, you were the lead author for the paper about HER2
testing that came out of an elite group from ASCO and CAP (Wolff
2007a, 2007b). Would you discuss the conclusions?
![]() DR WOLFF: The panel attempted to answer two questions. First, what is
the optimal testing algorithm for the assessment of HER2 status? Second,
what strategies can help ensure the optimal performance, interpretation and
reporting of assays? Essentially, clinical algorithms were established that
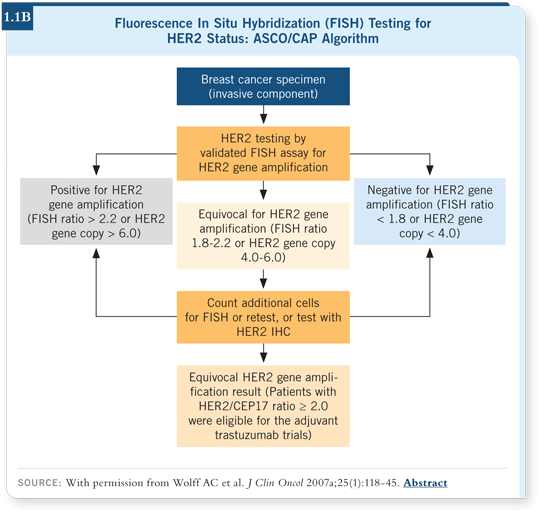
recapitulate what has been done in clinical practice (Wolff 2007a; [1.1A, B]).
DR WOLFF: The panel attempted to answer two questions. First, what is
the optimal testing algorithm for the assessment of HER2 status? Second,
what strategies can help ensure the optimal performance, interpretation and
reporting of assays? Essentially, clinical algorithms were established that
recapitulate what has been done in clinical practice (Wolff 2007a; [1.1A, B]).
The panel believed that, for the most part, no evidence supported the superiority of FISH compared to IHC. We decided to emphasize the importance of reflex testing for equivocal FISH results with IHC. We also formalized the definition of the equivocal range for FISH assays as a ratio of HER2 to CEP17 between 1.8 and 2.2 (Wolff 2007a; [1.1B]).

![]() DR WOLFF: We are also increasingly paying attention to the fixation of a
tumor specimen, a key issue that applies to other markers as well, such as ER.
The currently available HER2 assays have been validated using samples that
have been fixed in neutral buffered formalin between six and 48 hours. This
has huge implications from a practical standpoint for those women who have
their breast surgery on a Friday afternoon. The pathologist must continue
processing the tissue sample over the weekend, rather than waiting until
Monday morning.
DR WOLFF: We are also increasingly paying attention to the fixation of a
tumor specimen, a key issue that applies to other markers as well, such as ER.
The currently available HER2 assays have been validated using samples that
have been fixed in neutral buffered formalin between six and 48 hours. This
has huge implications from a practical standpoint for those women who have
their breast surgery on a Friday afternoon. The pathologist must continue
processing the tissue sample over the weekend, rather than waiting until
Monday morning.
![]() DR LOVE: What usually happens over the weekend? Could the tumor sit
without being placed in formalin?
DR LOVE: What usually happens over the weekend? Could the tumor sit
without being placed in formalin?
![]() DR ALLRED: We hope that doesn’t happen. The tumor usually is put in
formalin, but it isn’t sliced well. Sometimes it isn’t sliced at all. In a typical
scenario, the operating room nurse drops the entire piece of tissue in a big
bucket of formalin, but formalin can’t permeate within the tissue quickly.
Then the outside of the tumor is overfixed and the inside is underfixed.
DR ALLRED: We hope that doesn’t happen. The tumor usually is put in
formalin, but it isn’t sliced well. Sometimes it isn’t sliced at all. In a typical
scenario, the operating room nurse drops the entire piece of tissue in a big
bucket of formalin, but formalin can’t permeate within the tissue quickly.
Then the outside of the tumor is overfixed and the inside is underfixed.
![]() DR LOVE: Is there anything in the published literature about this?
DR LOVE: Is there anything in the published literature about this?
![]() DR WOLFF: Liz Hammond, who has a reference laboratory in Salt Lake City, presented a study about this
issue at the 2005 San Antonio
Breast Cancer Symposium.
She evaluated the prevalence
of ER-negative test results in
a homogeneous population
across seven facilities (Nkoy
2005; [1.2]).
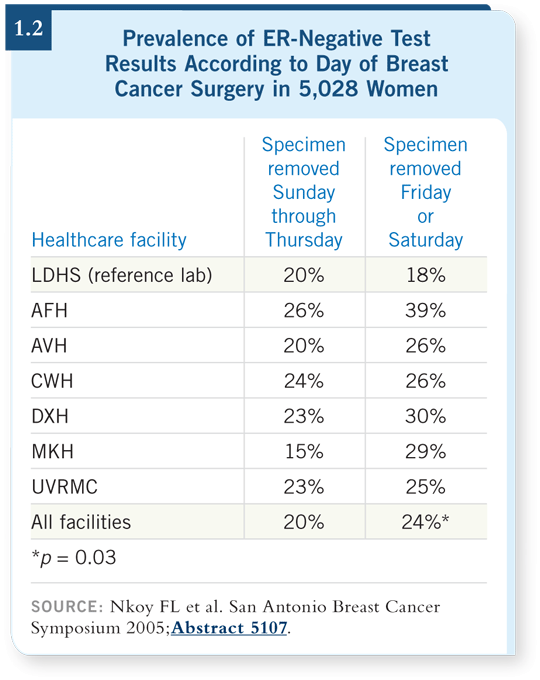
DR WOLFF: Liz Hammond, who has a reference laboratory in Salt Lake City, presented a study about this
issue at the 2005 San Antonio
Breast Cancer Symposium.
She evaluated the prevalence
of ER-negative test results in
a homogeneous population
across seven facilities (Nkoy
2005; [1.2]).
She reported by hospital and demonstrated variability, which indicates inconsistency in the time from tissue acquisition until it arrives in the laboratory for fixation. She also reported according to when the specimens were obtained. The specimens from the surgeries performed on Friday or Saturday had a lower prevalence of ER-positive results (Nkoy 2005; [1.2]).
Tracks 5-7, 9-10
![]() DR LOVE: Craig, what are some of the key issues related to quality
control with IHC and FISH for HER2?
DR LOVE: Craig, what are some of the key issues related to quality
control with IHC and FISH for HER2?
![]() DR ALLRED: It’s important to understand that both IHC and FISH are inherently
difficult tests to control. Even the experts have to work hard to keep
the sensitivity and reproducibility of the tests at an acceptable level. One
needs to know, what are those acceptable levels? How closely does your
laboratory reproduce the biological distribution of the test results? How do
the results vary from week to week, month to month, year to year?
DR ALLRED: It’s important to understand that both IHC and FISH are inherently
difficult tests to control. Even the experts have to work hard to keep
the sensitivity and reproducibility of the tests at an acceptable level. One
needs to know, what are those acceptable levels? How closely does your
laboratory reproduce the biological distribution of the test results? How do
the results vary from week to week, month to month, year to year?
Testing kits have been designed to remove most of the guesswork for the pathology laboratory in terms of the analytical and postanalytical or interpretive variables. But one must adhere closely to the recommended testing procedures, which is not always easy to do. In many laboratories, technicians don’t realize that a minor variation in the procedure can have a major effect on the test result. This is as true for FISH as it is for IHC.
![]() DR LOVE: Do we know where HER2 testing is being done?
DR LOVE: Do we know where HER2 testing is being done?
![]() DR WOLFF: I don’t have a sense of how much is being done by local laboratories
versus how much is being sent out. Community laboratories tend to
perform the IHC locally and send samples to a central laboratory for FISH testing. One of the proposed benefits of the new chromogenic assay is that you
can perform the in situ hybridization test in your own community lab.
DR WOLFF: I don’t have a sense of how much is being done by local laboratories
versus how much is being sent out. Community laboratories tend to
perform the IHC locally and send samples to a central laboratory for FISH testing. One of the proposed benefits of the new chromogenic assay is that you
can perform the in situ hybridization test in your own community lab.
Clinicians in the community assume that FISH is more accurate and more predictive, but nothing could be further from the truth. It has more to do with whether you’re performing the test correctly than whether you perform a particular test.
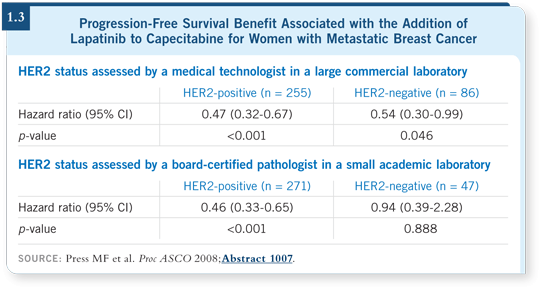
A perfect example was Michael Press’s experience with the recent study of capecitabine with or without lapatinib. When HER2 testing by FISH was done by a large commercial laboratory, a suggestion emerged that some patients with HER2-negative disease benefited from the addition of lapatinib. Michael retested all the samples in his own lab by FISH. He found that many of the tumors that were labeled HER2-negative by the central reference laboratory were in fact HER2-positive, which had led to the implication that patients with HER2-negative disease were benefiting from lapatinib (Press 2008; [1.3]).
When he investigated further, it appeared that most of the interpretation of the results of the FISH assay in the large commercial laboratory was not being done by a pathologist but by a technologist. His strong recommendation was that you need a pathologist not only to supervise the test but also to make the interpretation (Press 2008). I believe the key issue for the clinician in the community to remember is that simply because a tumor is tested with FISH doesn’t mean it is a better test.
![]() DR LOVE: How can a laboratory assure themselves that they are providing
accurate HER2 results? What should be the proportion of 2+ results with
IHC for a laboratory?
DR LOVE: How can a laboratory assure themselves that they are providing
accurate HER2 results? What should be the proportion of 2+ results with
IHC for a laboratory?
![]() DR ALLRED: As a clinician and a pathologist at a local institution, I would
want to convince myself that the distribution of results was within expected
range. One of the most sensitive indications is the proportion of 2+ results you
obtain with IHC.
DR ALLRED: As a clinician and a pathologist at a local institution, I would
want to convince myself that the distribution of results was within expected
range. One of the most sensitive indications is the proportion of 2+ results you
obtain with IHC.
Based on the data I have the most faith in, it’s probably about 10 to 20 percent. So I would say that 15 percent is the average rate for a 2+ result with IHC. That is a number that repeatedly comes out of expert academic laboratories as roughly correct. The frequency of 2+ results with IHC coming out of commercial laboratories varies from 10 to 60 percent. If you have around a 15 percent rate for 2+ results with IHC and you send the tumor for FISH analysis, if 30 to 50 percent come back amplified, you’re probably doing it right.
At the 2005 San Antonio Breast Cancer Symposium, two poster discussions dealt with FISH assay validation of HER2 2+ IHC results. One poster was from an academic laboratory, and it included approximately 2,000 patients. The other poster was from a large commercial laboratory, and it included approximately 10,000 patients.
The algorithm was for 2+ IHC — on which the initial screening was always based — to be sent for FISH analysis. In the academic laboratory, 15 percent of tumor samples went on to FISH, and around 30 percent were positive. In the commercial laboratory, 60 percent of the tumor samples were 2+ by IHC. Among the 60 percent, a far smaller proportion ended up being amplified by follow-up FISH.
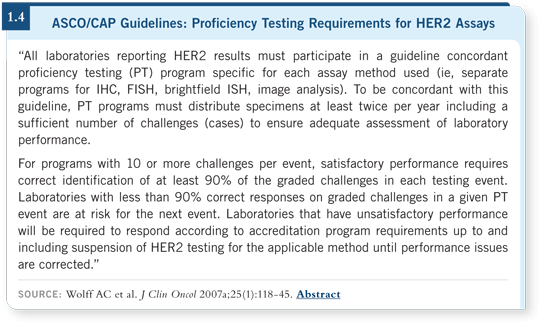
Next you should check your results against other laboratories and participate in the CAP accreditation programs, which have become much more rigorous, especially with the new ASCO/CAP guidelines (Wolff 2007a; [1.4]). Some pathologists, especially those in smaller institutions, use the word onerous rather than rigorous, because they don’t handle enough cases to meet the quality-control guidelines.
![]() DR SIMON: It would be nice to have national certification of HER2 testing
in individual laboratories. This would involve a central organization that
randomly sent out standardized materials with a known HER2 status and then
provided feedback regarding the sensitivity and specificity of HER2 testing in that laboratory. It would be voluntary on the part of the laboratory, but physicians
wouldn’t have to use laboratories that didn’t volunteer to participate in
national certification.
DR SIMON: It would be nice to have national certification of HER2 testing
in individual laboratories. This would involve a central organization that
randomly sent out standardized materials with a known HER2 status and then
provided feedback regarding the sensitivity and specificity of HER2 testing in that laboratory. It would be voluntary on the part of the laboratory, but physicians
wouldn’t have to use laboratories that didn’t volunteer to participate in
national certification.
Track 13
![]() DR LOVE: Joe, the Oncotype DX® assay is now reporting quantitative
HER2 results. What are the issues related to assessing HER2 status with
RT-PCR? What is actually being measured compared to when HER2 is
assessed with FISH and IHC?
DR LOVE: Joe, the Oncotype DX® assay is now reporting quantitative
HER2 results. What are the issues related to assessing HER2 status with
RT-PCR? What is actually being measured compared to when HER2 is
assessed with FISH and IHC?
![]() DR SPARANO: IHC measures protein expression. Gene amplification is
measured by FISH. In general, when FISH indicates gene amplification,
protein overexpression is almost always present.
DR SPARANO: IHC measures protein expression. Gene amplification is
measured by FISH. In general, when FISH indicates gene amplification,
protein overexpression is almost always present.
RT-PCR is a semiquantitative way of examining expression of RNA, the intermediary. It’s just another way of analyzing the same pathway. Information is emerging about the correlation between RNA expression and both gene amplification and protein overexpression.
![]() DR BUDD: We need correlation between RT-PCR and response to trastuzumab
in the setting of a randomized trial, which is forthcoming. Ultimately,
we’re trying to determine whether this test will predict response to a specific
therapy. The best way to evaluate the tests is in trials.
DR BUDD: We need correlation between RT-PCR and response to trastuzumab
in the setting of a randomized trial, which is forthcoming. Ultimately,
we’re trying to determine whether this test will predict response to a specific
therapy. The best way to evaluate the tests is in trials.
Track 18
![]() DR LOVE: How should the assessment of HER2 status be approached in
clinical practice?
DR LOVE: How should the assessment of HER2 status be approached in
clinical practice?
![]() DR SIMON: Today, a reasonable algorithm would be the following: If the
tumor is HER2-positive, then I go with that. If it is HER2-negative, then
I ask for it to be sent out a second time. From what I see, the downside of
having a false-negative result is greater than the downside of having a false-positive
one.
DR SIMON: Today, a reasonable algorithm would be the following: If the
tumor is HER2-positive, then I go with that. If it is HER2-negative, then
I ask for it to be sent out a second time. From what I see, the downside of
having a false-negative result is greater than the downside of having a false-positive
one.
![]() DR WOLFF: It actually goes both ways, because a false-positive result, which
occurred anywhere from 12 to 18 percent of the time in NCCTG-N9831
(Perez 2006), means you run the risk of potentially receiving a costly and
toxic placebo.
DR WOLFF: It actually goes both ways, because a false-positive result, which
occurred anywhere from 12 to 18 percent of the time in NCCTG-N9831
(Perez 2006), means you run the risk of potentially receiving a costly and
toxic placebo.
![]() DR SIMON: But if my tumor is HER2-positive, the treatment will benefit me.
If my tumor is HER2-negative, it won’t benefit me and I’ll be subject to some
toxicity. On the other hand, if my tumor is HER2-positive and it is actually
labeled HER2-negative, to have that drug withheld, I believe, is a greater
cost.
DR SIMON: But if my tumor is HER2-positive, the treatment will benefit me.
If my tumor is HER2-negative, it won’t benefit me and I’ll be subject to some
toxicity. On the other hand, if my tumor is HER2-positive and it is actually
labeled HER2-negative, to have that drug withheld, I believe, is a greater
cost.
![]() DR GOSS: However, $100,000 and a serious cardiac event for the wrong
reason are extremely important.
DR GOSS: However, $100,000 and a serious cardiac event for the wrong
reason are extremely important.
![]() DR LOVE: Also, many patients end up receiving chemotherapy because their
tumor is HER2-positive.
DR LOVE: Also, many patients end up receiving chemotherapy because their
tumor is HER2-positive.
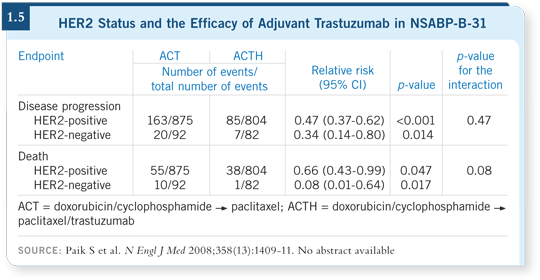
![]() DR SPARANO: We also have evidence that those patients with tumors that
are called HER2-positive by one laboratory and HER2-negative by another
laboratory may benefit from adjuvant trastuzumab (Paik 2008; [1.5]).
DR SPARANO: We also have evidence that those patients with tumors that
are called HER2-positive by one laboratory and HER2-negative by another
laboratory may benefit from adjuvant trastuzumab (Paik 2008; [1.5]).
TOPICS
Challenges in HER2 Testing and Interpretation
- Select publications
Assessment of Estrogen Receptor Status
- Select publications
Evolving Role of Genomic Assays in Breast Cancer
- Select publications
Tissue Biomarkers in the Management of Breast Cancer:
A CME Audio Series and Activity