| You are here: Home: BCU 6|2004: Robert B Livingston, MD
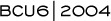
Robert B Livingston, MD |
EDITED COMMENTS |
 SWOG-S0221: Dose-dense versus “dose-denser” chemotherapy SWOG-S0221: Dose-dense versus “dose-denser” chemotherapy
Rationale for SWOG-S0221
The initial trial design of SWOG-S0221 was based on two small pilot studies that demonstrated that very dose-dense therapy for 20 to 24 weeks — with weekly doxorubicin and daily oral cyclophosphamide requiring G-CSF support — produced promising results in patients with node-positive disease. Patients with a median of four positive nodes had an 86 percent five-year disease-free survival, which compared favorably to the standard NSABP AC regimen in a similar population (2.1).
The results of CALGB-9741 were published in the Journal of Clinical Oncology in 2003, and that changed the landscape of clinical research in the adjuvant setting. Members of the Intergroup share a strong desire to build upon that trial, which showed the every two-week administration of AC and paclitaxel, with G-CSF support, was better than the every three-week schedule.
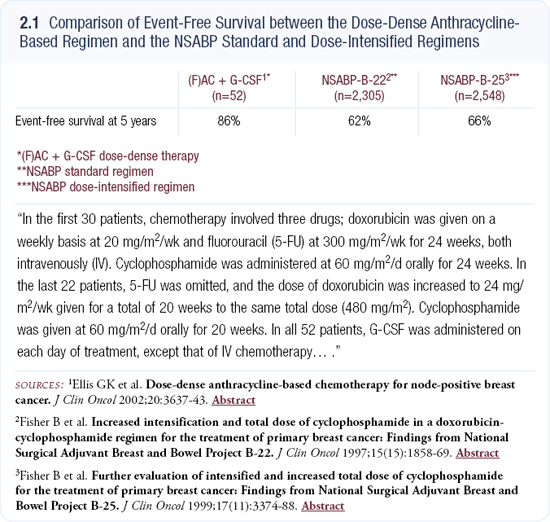
The logical next step would be a comparison of every two-week AC and our weekly doxorubicin and daily cyclophosphamide regimen — “dose-dense versus dose-denser.” The evaluation of weekly paclitaxel was suggested by the outcome of the MD Anderson neoadjuvant study (2.2), which randomly assigned patients to every three-week versus weekly paclitaxel, with the FAC component constant in both arms. A major advantage was seen in the pathologic complete response — 28 versus 14 percent — for patients who received weekly paclitaxel.
SWOG-S0221 study design
The study design was changed to preserve the design of CALGB-9741, but modified to examine the ultimate dose-densification schedule that is practical. Randomization includes four possible treatment options: every two-week AC or continuous AC, each followed by either every two-week or weekly paclitaxel.
Growth factor support is used in each arm of the trial. Pegfilgrastim — the pegylated form of G-CSF — is utilized in the every two-week arms, and patients treated with the weekly doxorubicin and daily cyclophosphamide regimen will receive filgrastim because we do not have experience with pegfilgrastim and concurrent chemotherapy and the FDA will not allow it.
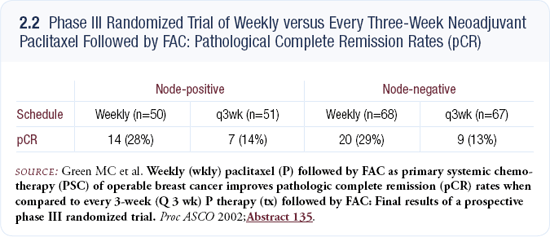
The study is a two-by-two factorial design (2.3). We will not have enough statistical power to formally test for superiority of each of the four arms, but we have more than enough power to test for the weekly versus every two-week approaches, which was the same statistical approach taken in CALGB-9741. The study will accrue approximately 4,500 patients, which is almost twice as many as CALGB-9741.
Tolerability of continuous AC plus G-CSF
In general, continuous AC plus G-CSF is much better tolerated than intermittent AC with much less nausea and vomiting. Every time I speak with physicians who have used it for a few months, they tell me, “I can’t believe it. Patients just aren’t getting sick.” That’s not quite true. They have some nausea, but generally it’s the nausea associated with a morning dose of cyclophosphamide, and usually you can take care of it by prescribing prochlorperazine. The fatigue is much less severe but more continuous.
One side effect of continuous AC plus G-CSF that is not typically seen with AC at standard doses is hand-foot syndrome. With 12 weeks of therapy, approximately 10 percent of patients will have Grade II hand-foot syndrome. I think it reflects epithelial cell damage from a proliferating compartment that has a relatively low turnover compared to the bone marrow.
Hand-foot syndrome is managed in the same manner as in a patient receiving capecitabine or continuous infusion 5-FU — discontinue the anthracycline for one week. The symptoms of hand-foot syndrome are typically much improved and you can resume at a reduced dose; we usually reduce the dose by 25 percent.
Incorporation of pegfilgrastim into dose-dense schedules
Studies performed with standard regimens given every three weeks or every two weeks demonstrated pegfilgrastim is equivalent to filgrastim in maintaining neutrophil counts. The toxicities are comparable and generally consist of bone pain resulting from the rapid expansion of the bone marrow.
The FDA’s point of view seems to be that filgrastim and pegfilgrastim are different drugs, and one should not assume pegfilgrastim can be administered concurrently with chemotherapy and maintain effective concentrations of the chemotherapy. I think this is a shortsighted view, and in the long run it’s going to slow the progress of clinical research. However, I’m certainly not in a position to counter the FDA and recommend that people administer pegfilgrastim concurrently with chemotherapy.
When doxorubicin is administered on a weekly basis, the levels of the agent are therapeutically effective for three to four days, and cyclophosphamide has a half-life of 12 hours for the activated metabolites. In our pilot studies we have treated over 300 patients.
We have been giving G-CSF concurrently with concentrations of these drugs even though we avoided the administration of G-CSF on the same day as the doxorubicin. I think one of the major concerns expressed by the FDA is that because pegfilgrastim is present for about 11 days, if you have a weekly treatment program, pegfilgrastim will obviously still be present when you administer the second dose of doxorubicin.
Two theoretical concerns exist. Pegfilgrastim may stimulate bone marrow stem cells that could then be affected by a DNA-damaging agent, which would result in a greater incidence of acute leukemia. The second concern is that filgrastim and pegfilgrastim may have different toxicities.
Leukemia secondary to dose-dense adjuvant chemotherapy
In the Ellis pilot trials evaluating (F)AC + G-CSF, one patient developed acute myeloid leukemia — and she had the characteristic translocation for an anthracycline- associated leukemia. Over 300 patients who received this regimen have now been followed for a median of four years, and only this one patient developed leukemia.
In addition, the Southwest Oncology Group did a neoadjuvant trial of over 100 patients, also chaired by Dr Ellis, in which the median follow-up is now approximately three and a half years, with no cases of acute leukemia. Conservatively, one can say that the incidence of acute leukemia observed with this regimen will not be greater than the incidence of acute leukemia one would expect with the same regimen given without growth factor support.
If you look at the MD Anderson database Anderson database at 10 years follow-up, the expected frequency of acute leukemia after the administration of doxorubicin for patients who survive 10 years is about one percent. The leukemogenic risk may actually be lower with regimens that administer lower individual doses of the drug.
The NSABP experience suggests that the risk of acute leukemia may be related to peak blood levels. They conducted studies in which the dose of cyclophosphamide ranged from 600 to 1,200 to 2,400 mg/m2, and those studies showed a higher incidence of leukemia in the patients receiving higher doses of cyclophosphamide.
At the time, some physicians speculated that it was related to the use of G-CSF in the higher-dose arms, but I think it was probably related to the presence of higher peak chemotherapy concentrations. We know that higher peak concentrations of alkylating agents are likely to be associated with development of leukemia.
Anthracycline-related leukemias tend to occur relatively early — between 12 and 36 months after treatment — so if you have a median follow-up of four years, you can be reasonably confident making a statement regarding the incidence of those leukemias. Leukemias related to alkylating agents are typically spread over a much longer period of time and continue to occur throughout a 10-year time period.
Counseling patients about the risk of leukemia from adjuvant chemotherapy
I tell every patient who will receive an anthracycline, “You probably have a lifetime risk for developing acute leukemia of about one percent as a result of this treatment.”
The risk of acute leukemia from alkylating agents depends on the agent utilized and the way it’s administered. If you look at CMF (cyclophosphamide, methotrexate and 5-FU) with oral cyclophosphamide — analogous to our AC program — the incidence of acute leukemia is no higher in more than 20 years of follow-up than in the women who received Bonadonna CMF.
No evidence exists to indicate that daily oral cyclophosphamide given for six months or cyclophosphamide given two weeks on and two weeks off for six months is more leukemogenic than no therapy.
Rationale for the effectiveness of dose-dense scheduling
The results of CALGB-9741 support the basic hypothesis I’ve had since the late 1980s, which is if you achieve a critical concentration necessary for cell kill, you’re more likely to get an effective result in direct proportion to the amount of time, or area under the curve, that the tumor cells are exposed.
If you administer doxorubicin once a week, tumor cells are exposed at least 50 percent of the time. If you give doxorubicin every two weeks, they’re exposed about three to four days out of every two weeks. If you give it once every three weeks, the tumor cells are exposed for three or four days every three weeks.
That may sound a little simple-minded, and the explanation is probably more complex, but I think the exposure of cells to effective concentrations of chemotherapy over a longer period of time is the key to why dose-dense therapies work better.
A second reason, which may be very important, is the antiangiogenic hypothesis. We now have good preclinical data that demonstrate that with continuous exposure, certain classes of agents — cyclophosphamide, the vincas and the taxanes — result in much better cell kill and tumor regressions than intermittent exposure. There is solid evidence in preclinical systems that an antiangiogenic effect is the primary reason for that cell kill.
Optimizing adjuvant doxorubicin/cyclophosphamide
With weekly therapy, I think we have a pretty good handle on the effect of dose reductions, mostly from the Europeans. For doxorubicin, if you don’t deliver 15 mg/m2 per week, at least in the setting of advanced disease, response rate goes down, and I think response rate is a crude surrogate for cell kill.
One of the main reasons we incorporated filgrastim early on was that my colleague, Dr Ellis, and I initially did a trial in which we gave continuous AC (15 mg/m2 per week of doxorubicin) without growth factor support. Only about 15 percent of patients were able to tolerate the intended dose. With growth factor support, approximately 90 percent of patients receive the intendeddose.
Every three-week AC is an outmoded regimen. If AC is utilized, it should be given every two weeks with growth factor support. Again, you have to realize that my treatment approach is different from many other physicians because I use CMF more frequently than AC.
For a patient for whom I’m worried enough about risk factors to think she needs an anthracycline-based regimen, I would use the every two-week schedule of AC with growth factor support.
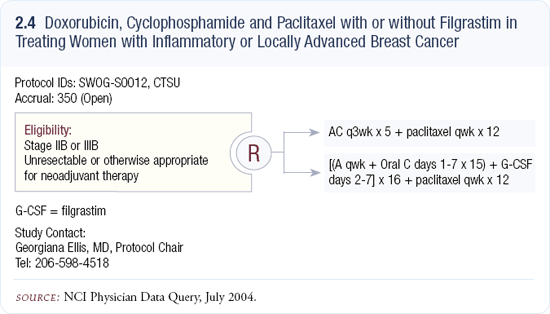
SWOG trial S0012 of neoadjuvant therapy in locally advanced and inflammatory disease
In the Southwest Oncology Group we have a trial of neoadjuvant therapy for women with locally advanced and inflammatory disease, comparing intermittent AC versus AC plus G-CSF (2.4). That trial is accruing reasonably well. All patients receive paclitaxel, but it’s a two-arm study and paclitaxel is administered weekly for 12 weeks.
I would like to see an Intergroup trial in which patients who have resectable disease but want to receive neoadjuvant therapy are randomly assigned to a dose-dense versus a less dose-dense schedule. In other words, a trial asking the same basic question that we’re asking in SWOG-S0221, because with an endpoint of pathologic complete response in a two-arm design, we could potentially have an answer in a couple of years while we’re still completing the adjuvant study.
Incorporation of capecitabine into regimens with metronomic scheduling
Capecitabine is a drug that meets the criteria for dose density. It’s administered two weeks out of three, which is not continuous but it’s close. If the current Intergroup trial shows an advantage for continuous versus every two-week therapy, I would favor seeing the next study evaluate the addition of capecitabine.
I think we would want to add capecitabine to a taxane, not to the AC regimen, because if you add capecitabine to AC, you’re going to see the same thing we saw in the initial studies when we added 5-FU. Our original study was with FAC plus G-CSF, and hand-foot syndrome occurred in 70 percent of the patients. You could add capecitabine to AC every two weeks, or you could add it to the paclitaxel at the back end.
Select publications
 |
Dr Livingston is a Professor of Medicine and Oncology at the Seattle Cancer Care Alliance in Seattle, Washington. |
|
