| You are here: Home: BCU 6|2004: Daniel F Hayes, MD
Daniel F Hayes, MD |
EDITED COMMENTS |
 Genetic profiling to predict prognosis Genetic profiling to predict prognosis
Currently, 75 percent of our patients have node-negative tumors of which nearly 80 percent are ER-positive. At best, adjuvant chemotherapy improves survival in this group by two or three percent over a 10-year period. Should we treat 100 patients in order to improve the survival of three? If you’re one of the three, the answer is yes. It would be much more efficient if we could identify and treat only the patients in whom the disease is likely to recur.
The NSABP has partnered with Genomic Health Inc to develop a multiplex RT-PCR system that can analyze up to 300 genes at a time. In three preliminary studies, they were able to narrow it down to approximately 20 genes that appeared to be prognostic in patients with node-negative, ER-positive tumors who received tamoxifen. It was then tested prospectively in the tamoxifen arm of NSABP-B-14, and three categories of patients were successfully identified. They were able to profile 99 percent of the 600 or 700 specimens they analyzed, which indicates this is a very robust assay that works even on archived tissues.
At 10 years, 51 percent of the patients had a favorable prognostic profile, 22 percent had an intermediate profile and the remaining 27 percent had a poor profile, with recurrence rates of seven percent, 14 percent and 30 percent, respectively. I struggle with whether or not to recommend chemotherapy to these patients, but if this data is accurate I can tell at least half of them that their prognosis is so good that chemotherapy is not indicated.
We don’t know whether the assay used in B-14 would have the same effect in women with node-negative, ER-positive disease who received adjuvant anastrozole because it hasn’t been tested, but their prognosis is at least as good as those who received tamoxifen, so I cannot imagine it would not be applicable. Although I don’t know this for certain, I believe it’s likely this assay will be applied to tissues collected in the ATAC study.
I co-chair the American Society of Clinical Oncology Tumor Marker Guidelines Panel, which has established a very conservative group of recommendations because most of the tumor marker studies have been conducted without any forethought as to how patients were treated, how the samples were selected or how they’re processed. On the contrary, the assay generated by NSABP, in collaboration with Genomic Health, was studied the way I believe a tumor marker should be.
Prospective Intergroup study stratifying patients by risk based on tumor genetic profile
The Intergroup is designing a prospective study that will use the Genomic Health assay to prospectively stratify patients with node-negative, ER-positive disease into three prognostic groups: good, intermediate and poor. All patients will receive tamoxifen, patients with a poor profile will also receive chemotherapy, and patients in the intermediate group will be randomly assigned to chemotherapy or no chemotherapy.
George Sledge is designing this trial, which will be led by ECOG and probably will include every cooperative group in North America. The trial design is not yet finalized, but if it proceeds as described, it will probably be the last prospective trial of chemotherapy versus no chemotherapy in the adjuvant setting.
Randomization of the patients in the intermediate group will determine whether this assay can identify predictive factors for this group and which patients are most likely to benefit from the chemotherapy.
Predicting pathologic response to chemotherapy based on genetic profiling in the neoadjuvant setting
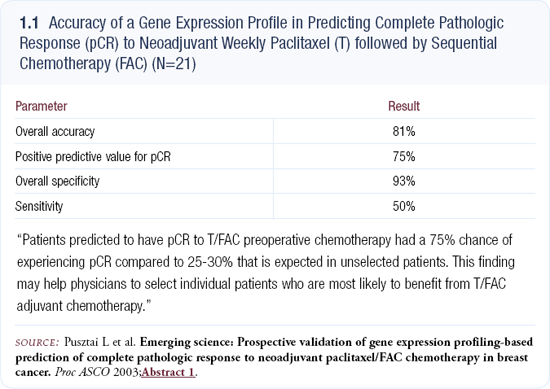
At ASCO 2003, Lajos Pusztai and his colleagues reported on a preliminary study suggesting they could identify patients most likely to have a complete pathologic response to combination chemotherapy based on gene expression profiling (1.1).
Similarly, two or three other studies, including work conducted at Georgetown, suggest that not only can general resistance to all chemotherapies be predicted, but resistance to single agents in neoadjuvant therapy — such as a taxane versus doxorubicin — can also be predicted.
This research is very much in its infancy, and Dr Pusztai will chair a SWOG neoadjuvant trial with fine-needle aspiration before treatment to confirm his preliminary findings.
While Dr Pusztai’s study evaluated combination chemotherapy, we know that cyclophosphamide, doxorubicin and 5-FU work in very different ways. Logic tells us we’ll probably find that some genes are associated with resistance to all chemotherapy and other genes are specific for individual drugs.
For a long time we have fantasized about being able to individualize therapy based on a patient’s genes, but I believe we’re beginning to develop the tools and the technology to do just that.
Pharmacogenomics and pharmacogenetics
This field is about to explode. This field involves the study of genes and how they predict response to drugs. For example, we all have the same genes that metabolize drugs in our liver, but each patient has a slightly different set of alleles. Two patients may take the same drug but they probably metabolize it differently. For more than 100 years we’ve known that metabolism varies, but now we have the genetic tools to begin to understand it.
The NIH has funded a large consortium of experts to examine various drugs. Mark Ratain has received funding to evaluate chemotherapeutic agents. We have been examining whether we can use a patient’s phenotype to determine the appropriate dose of chemotherapy.
Anne Schott conducted a study with docetaxel, which is metabolized by the same gene that metabolizes erythromycin. Patients received an injection of erythromycin, and then their phenotypes were established via a breath test to determine how quickly they metabolized the drug.
Patients were then given a dose of docetaxel based on their metabolic phenotype and their body surface area. I participated in this trial and some patients received doses a lot higher than normal and did fine, while other patients received doses much lower than normal and experienced toxicities.
SWOG and most of the major cooperative groups are planning large-scale correlative studies of pharmacogenomics. We’re trying to collect and bank white cell DNA and tumor specimens to examine single nucleotide polymorphisms in various genes that may be important for metabolism to see if we can determine who will experience toxicities and, perhaps, who will benefit from therapies. I encourage clinicians to support this important work. I believe a major breakthrough will occur in this field, and these banks will be gold mines in the future.
Proposed SWOG trial evaluating continued trastuzumab after progression on trastuzumab and a taxane
The trial will randomly assign patients to vinorelbine or vinorelbine plus continued trastuzumab. We need to determine if continuing trastuzumab in this setting is beneficial. Theoretically, it shouldn’t be unless there’s synergy, which has been suggested by Dennis Slamon’s work. Dr Pusztai will chair this study, which began at MD Anderson and is being adopted by the Intergroup.
This may be the last trial in the metastatic setting to randomly assign patients to trastuzumab, so it’s an ideal place to look for a predictive factor. In this study, we’ll examine circulating tumor cells and HER2 expression on the cells to determine whether we can predict which patients will benefit from or be resistant to trastuzumab.
This study is important because we don’t know the value of continuing trastuzumab after progression. In my practice, when a patient progresses on trastuzumab, I have a mixed approach. Occasionally I continue the drug, but I’d like evidence that it’s beneficial.
Treatment of patients following progression on hormonal therapy
Patients on hormonal therapy may have tumors that have become relatively resistant to specific hormonal agents, like SERMS. Because of evidence that cross talk occurs in the EGFR family (especially between HER2 and ER), combining an agent like trastuzumab with tamoxifen may effectively overcome resistance of a previously resistant drug and produce better results. Several attempts have been made to mount randomized trials to determine if this is true, but they’re difficult to conduct in the adjuvant setting because most of these patients are on tamoxifen when they relapse.
We can’t randomly assign patients with progressive disease on tamoxifen to continuing tamoxifen alone versus tamoxifen plus trastuzumab, because the control arm is unethical. One trial attempted to randomly assign patients to trastuzumab with or without tamoxifen, but it was not very practical and that trial has been aborted.
In my practice, I use an aromatase inhibitor when a patient progresses on adjuvant tamoxifen, assuming the patient does not have rapidly progressing visceral disease that might prompt me to start chemotherapy immediately. I struggle with how to treat premenopausal patients, but generally suggest ovarian ablation, either surgically or with an LHRH antagonist.
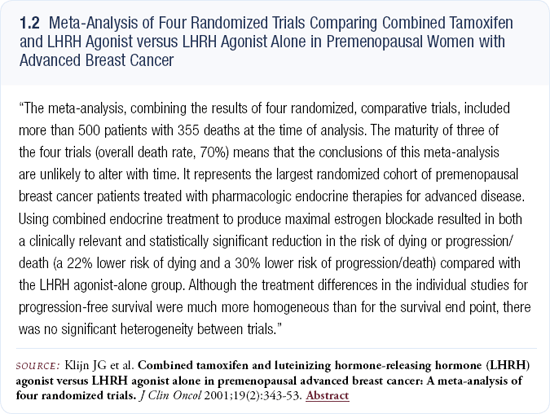
When I use an LHRH antagonist, I tend to add an aromatase inhibitor because Klijn’s meta-analysis suggests that combination hormonal therapy is probably superior to single agents (1.2). It’s one of the few instances in which I believe combination endocrine therapy makes sense.
Combination versus single-agent endocrine therapy
Many studies have evaluated combination versus single-agent endocrine therapy. In almost every case, the response rates to the combinations are higher, but in most cases, the survival rates are almost identical. The toxicities are also higher with combination therapy, so I tend to use sequential endocrine therapy. I don’t know what to make of the statistic “duration of response.”
The important endpoint for endocrine studies in the metastatic setting is the length of time until chemotherapy is needed, because we’re trying to palliate these patients. To my knowledge, this has almost never been evaluated, although obviously, the longer the patient is in response, the longer the interval before they need chemotherapy.
Management of premenopausal patients with ER-positive disease
Data suggest that in premenopausal patients with ER-positive metastatic disease, ovarian ablation plus an aromatase inhibitor results in a small prolongation of survival compared to ovarian ablation alone. In the adjuvant setting, no one really knows how to treat women under the age of 40 with ER-positive disease who continue to menstruate after chemotherapy. Some physicians believe they should undergo ovarian ablation and receive tamoxifen, some believe their ovaries should be ablated and they should receive aromatase inhibitors, and others believe tamoxifen alone is satisfactory.
Three randomized trials in premenopausal patients are being opened in Western Europe, North America and in the international Intergroup. Investigators will be able to choose one or more studies to fit their bias. For example, if they believe premenopausal women should undergo ovarian ablation after chemotherapy, they can choose a study evaluating aromatase inhibitors versus tamoxifen, or if they’re not sure about ovarian ablation, they can choose a study in which young women will receive tamoxifen with or without ovarian ablation. I believe we’ll have an answer in the next few years.
In the metastatic setting, I tend to add an aromatase inhibitor to ovarian ablation in premenopausal patients because the complications of estrogenopenia are less concerning — unfortunately, most patients are not going to survive long enough for this to be an issue.
However, in the adjuvant setting, especially in a group of patients with a relatively good prognosis, combining these agents may cause substantial health consequences. I support these randomized trials because they compare the competing morbidity of long-term estrogen depletion to the risk of the breast cancer.
Fulvestrant in the treatment of metastatic disease
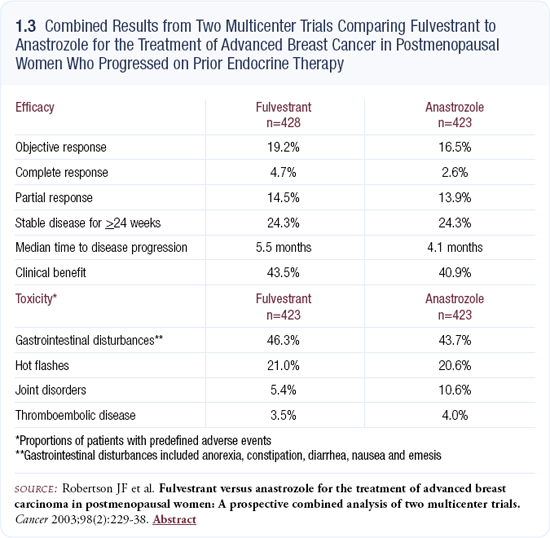
I use fulvestrant as third-line therapy in patients whose disease has progressed on tamoxifen and an aromatase inhibitor. That’s the current indication, but it wouldn’t surprise me to see it moved up because data from the randomized trials clearly suggest it is as effective as aromatase inhibitors in patients who progressed after tamoxifen (1.3). The clinical question is whether the patient prefers a pill versus parenteral injection. For some patients, the injection is easier, but most patients prefer taking a pill. In my experience, the tolerability of fulvestrant is similar to that of the aromatase inhibitors.
SWOG trial comparing combination versus single-agent hormonal therapy
SWOG is about to initiate a study in which patients will be randomly assigned to anastrozole with or without fulvestrant. We need to determine whether it’s better to give these agents sequentially or in combination, and I’m hopeful we can measure the length of time until the patient needs chemotherapy.
Based on historical data and my own clinical experience, I expect sequential single-agent therapy will be just as effective as the combination and will have fewer side effects. However, I am supportive of this trial and will enroll patients willingly because if it turns out I’m wrong, then we’ve made a step forward.
The combination may result in better outcomes for biological reasons. By depleting estrogen levels as low as possible, and then using an estrogen receptor downregulator, we’re doing more than just preventing estrogen from getting into the cells. It’s like putting water in the gas tank — we not only prevent the estrogen from getting in, but we damage the engine as well.
A secondary endpoint will be response rates in patients who receive anastrozole followed by fulvestrant. Responses have been shown in retrospective analyses of trials, but this will more precisely measure how often responses occur. We’re also going to perform a number of correlative science studies looking at the HER2 and EGFR pathways and interactions to see if we can identify a group of patients who might benefit more from the combination than from sequential single agents.
Neoadjuvant chemotherapy
Neoadjuvant chemotherapy is an exciting area of research. In terms of outcome, it doesn’t matter whether adjuvant chemotherapy is given before or after surgery, but giving it preoperatively may offer some benefits. Obviously, neoadjuvant therapy can increase the chances of breast preservation, but it may also be useful in individualizing therapy. By using serial biopsies and genomics, we may be able to identify futile therapies and switch to another therapy earlier.
In NSABP-B-27, all patients received AC and were randomly assigned to one of three arms: surgery, surgery followed by docetaxel, or docetaxel followed by surgery. The question is whether we can identify patients whose response to AC alone is sufficient and their risk is too low to warrant further adjuvant chemotherapy. Perhaps we can identify patients who are resistant to all therapies, in which case further chemotherapy is not indicated.
In the next 10 years neoadjuvant trials will be designed to individualize therapy. Genomics and proteomics will be used to examine tumor profiles and evaluate how patients respond to therapies. The patient’s pharmacogenomic profile and the presence of micrometastatic disease may be utilized to select a therapeutic regimen that is specific to her needs.
Select publications
 |
Dr Hayes is a Professor of Internal Medicine and Clinical Director of the Breast Oncology Program in the Department of Internal Medicine’s Division of Hematology/Oncology at the University of Michigan Comprehensive Cancer Center in Ann Arbor, Michigan. |
|
