| You are here: Home: BCU 6|2004: Mitchell Dowsett, PhD
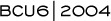
Mitchell Dowsett, PhD |
EDITED COMMENTS |
 IMPACT neoadjuvant trial IMPACT neoadjuvant trial
The IMPACT trial compared anastrozole, tamoxifen and a combination of the two as neoadjuvant therapy in postmenopausal women with ER-positive tumors that were more than two centimeters. Initially, Ki67 was our primary endpoint; however, we turned the study into a larger trial with clinical endpoints. We tried to base the study on the ATAC trial and used a placebo-controlled, double-blind design — neither the patients nor the surgeons knew what the patients were receiving.
Clinical outcomes
At the last San Antonio Breast Cancer Symposium, my colleague, Ian Smith, presented the clinical outcomes data from the 330 patients enrolled (3.1). In the intent-to-treat analysis for clinical response, no difference was found between anastrozole, tamoxifen and the combination. In the women requiring mastectomy at baseline, anastrozole demonstrated a significant advantage over tamoxifen in terms of rendering the women eligible for breast-conserving surgery — between 40 and 50 percent of the women in the anastrozole arm and just over 20 percent in the tamoxifen arm.
In a previous neoadjuvant trial comparing an aromatase inhibitor to tamoxifen, letrozole was used. In that particular study, all of the patients required mastectomy at baseline. We felt it was important to compare our study’s results with that letrozole study.
For some biologically and clinically interesting reason, patients requiring mastectomy seem to do better with an aromatase inhibitor than with tamoxifen. It would be great to find out why the aromatase inhibitors have greater antitumor effect in these larger tumors.
My clinical colleagues remind me that clinical response is a soft endpoint and, particularly in smaller tumors, it’s difficult to measure small changes between a three-centimeter and a two-centimeter tumor. Clearly, in patients requiring mastectomy, the tumors are much larger; therefore, an error in establishing and measuring response is less likely. We had hoped that the clinical response in the IMPACT trial would be a surrogate endpoint for the outcomes in the ATAC trial, which demonstrated that adjuvant anastrozole was better than tamoxifen or the combination at increasing relapse-free survival. In essence, however, clinical response was not a good surrogate.
Biomarkers
As a translational scientist, my attraction to neoadjuvant trials exists because pretreatment biopsy material is obtainable. In this circumstance the second specimens are particularly valuable. Indeed, we had specimens from about 156 patients who were evaluable at two weeks, and we compared them with the 230 to 240 patients with specimens taken pretreatment and at three months.
We evaluated a number of biomarkers — apoptosis, ER, PR, HER2 and EGFR. However, we focused on Ki67 — a marker of those cells that are actively cycling. We’ve done many of these types of studies over the years, but this is the largest. It was also the most important study because it gave us the opportunity to ask: Could the change in Ki67 actually predict the outcome of the ATAC trial?
It was a delight to be able to report that the outcome with the biomarker was comparable to the outcome of the ATAC trial. The reduction in Ki67 associated with neoadjuvant anastrozole was just below 80 percent at two weeks, and that reduction increased marginally to 82 percent at three months.
For neoadjuvant tamoxifen, the reduction in Ki67 at two weeks was about 60 percent. Both at two weeks (p = 0.004) and at three months (<0.001), the reduction in Ki67 was significantly less for tamoxifen than for anastrozole. The combination arm at two weeks and at three months performed exactly the same as the tamoxifen arm.
Some other provocative observations became evident when we evaluated the detail of the changes in Ki67. In the anastrozole arm, only three or four out of over 50 patients did not show a numerical reduction in proliferation after two weeks. This suggests that probably over 90 percent of patients show some sort of biological response to anastrozole.
In the tamoxifen arm, eight out of 50 patients did not show a reduction in Ki67, and the overall reductions were smaller. Perhaps only half of the patients with ER-positive disease are clinically responsive, but biologically they appear much more responsive. Our overall conclusion from the IMPACT trial was that the changes in Ki67 are probably a better surrogate marker for the benefit from these drugs than clinical response.
Results of the IMPACT trial and HER2 status
HER2 was an interesting marker to evaluate in the IMPACT trial. In the patients with disease that was both ER- and HER2-positive, we saw a 58 percent response rate with anastrozole and a 21 percent response rate with tamoxifen. Given the very small numbers of trial participants with ER- and HER2-positive disease, the difference wasn’t statistically significant.
These data are comparable to the data reported by Matt Ellis, demonstrating that neoadjuvant letrozole had a markedly better response rate than tamoxifen in patients with either HER1- or HER2-positive disease. This substantiates that in the neoadjuvant setting, patients with ER- and HER2-positive disease respond better to an aromatase inhibitor than to tamoxifen.
Planned neoadjuvant trial of anastrozole and gefitinib
In our next trial, we’ll be incorporating a tyrosine kinase inhibitor with an endocrine agent, and Ki67 will be our primary endpoint. The trial has a slightly complicated but novel design. Initially, all 180 patients will receive anastrozole alone for two weeks; then they will be randomly assigned to gefitinib or placebo. The patients will be treated for three months.
This design allows for a biopsy at two weeks to determine whether the response to gefitinib will be greater in patients who are not having a substantial reduction in Ki67 with anastrozole. The hypothesis is that we will see enhanced suppression of Ki67 — particularly in patients with little or no change in Ki67 while on anastrozole alone because that is the biologically refractory group. We believe that patients who aren’t responding well to an endocrine agent are the most likely to benefit from an agent that inhibits growth factor receptors.
Influence of endocrine therapy on the progesterone receptor
In a previous study published in the Journal of Clinical Oncology, we compared vorozole and tamoxifen. The aromatase inhibitor vorozole produced a very rapid and substantial fall in PR levels. Since the PR gene is exquisitely estrogensensitive, that’s not a surprise. After two weeks of tamoxifen, we actually saw an increase in PR levels in most patients.
Even at three months, when the PR levels began to fall, they still didn’t go below the baseline level. We see this as one of the clearest indications that tamoxifen has a substantial agonist effect, at least on the PR gene. We believe that is one of the key reasons the aromatase inhibitors are more beneficial than tamoxifen.
ATAC adjuvant trial: Subgroup analysis of patients with ER-positive, PR-negative disease
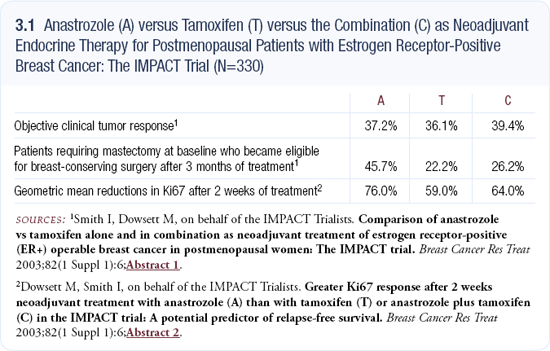
The ATAC trial enrolled 9,366 patients, and the first report demonstrated a significant benefit for the patients with hormone receptor-positive disease who were treated with anastrozole compared to tamoxifen.
The hazard ratio for disease-free survival in this group was 0.78. The 47-month analysis had a similar hazard ratio. Because the ATAC trial was designed in 1994 and initiated in 1996, it didn’t require the patients to have ER- and/or PR-positive disease for enrollment.
Hence, a very small proportion of patients had ER- and PR-negative disease, and a larger cohort had ER- or PR-unknown disease. We retrospectively analyzed the histological blocks from those patients for their ER and PR status to obtain a more comprehensive view of the influence of the ER and PR status on the outcomes of the trial. We asked whether the PR status had any impact on the relative benefit associated with anastrozole and tamoxifen in patients with ER-positive disease.
In the patients with ER- and PR-positive disease, which consisted of approximately 5,700 patients, anastrozole was more beneficial than tamoxifen, with a hazard ratio of 0.82. In the patients with ER-positive and PR-negative disease, a very substantial difference was noted, with a hazard ratio of 0.48, indicating that patients treated with adjuvant anastrozole had half as many relapses as patients treated with adjuvant tamoxifen (3.2).
The comparison between patients with ER- and PR-positive disease to patients with ER-positive and PR-negative disease was borderline for statistical significance. Although this was a retrospective subgroup analysis, I hope that other aromatase inhibitor trials will perform the same analyses to substantiate this finding.
Trials evaluating sequential adjuvant hormonal therapy
The MA17 trial data are exciting but controversial because the trial was stopped early. Irrespective, we have data indicating that the relapse rate in patients who had taken five years of adjuvant tamoxifen was reduced by about 50 percent with the introduction of letrozole. Because the trial was stopped early, we won’t be able to determine whether a survival benefit exists as well.
Does tamoxifen over a five-year period sensitize micrometastases to the influence of the aromatase inhibitors? As a translational scientist, I wonder if we could identify the patients at highest risk for relapse. The collaborators in the MA17 trial are addressing this question.
In the MA17 trial, it would be fascinating to determine whether the patients who are at the greatest risk for relapse after five years of adjuvant tamoxifen and would benefit most from the aromatase inhibitor are, indeed, those with ERpositive and PR-negative disease. They have the potential to perform that study very soon, because 98 percent of the ER and PR data has already been collected.
The Italian trial by Boccardo, in patients treated with adjuvant tamoxifen for two years followed by adjuvant anastrozole for three years, clearly demonstrates a significant benefit for switching to the aromatase inhibitor, but I believe the data are premature. The MA17 trial enrolled thousands of patients, but the Italian trial only enrolled a few hundred patients.
Biological rationale for the sequencing of adjuvant hormonal therapy
If the ATAC trial data from the patients with ER-positive and PR-negative disease were confirmed, it would be difficult to substantiate the use of adjuvant tamoxifen followed by adjuvant letrozole in that group of patients.
The relapse rate was too high with adjuvant tamoxifen to suggest such a sequential strategy, and it may be best to use an aromatase inhibitor early in that group of patients.
In the patients with ER- and PR-positive disease, in whom the relapse rates for tamoxifen and anastrozole were more similar, one could argue for the use of such a sequential strategy. However, I suspect even in that group of patients it is best to accept the gain associated with the aromatase inhibitors as initial adjuvant therapy, rather than allow a few patients to relapse and have to treat their metastatic disease.
Mechanisms of resistance in estrogen-deprived breast cancer cells
We have a series of preclinical models in which we’ve been investigating the mechanisms of resistance to estrogen deprivation. Cells that are estrogen-deprived for a short time become quiescent, but if we keep them in that environment for about 20 weeks without any further perturbation, they begin to grow again. This is similar to the patient who’s receiving an aromatase inhibitor and then becomes resistant to it.
Over the years we have considered this to be estrogen independence. Richard Santen and his colleagues have substantiated our evidence that it’s due to estrogen hypersensitivity — the cells grow in response to the very small amount of residual estrogen in the cell culture medium. We have asked: What made these cells hypersensitive? Again we came back to the growth factor receptors. In these cells, we see HER2 is overexpressed, the ER is phosphorylated and active, and the PR levels are increased.
What can we do about it? We’ve considered utilizing fulvestrant, a pure antiestrogen. In patients with estrogen hypersensitivity, we have observed that fulvestrant is effective and tamoxifen is not. Two clinical trials — the Evaluation of Fulvestrant versus Exemestane Clinical Trial (EFECT) and the Study of Fulvestrant versus Exemestane with/without Anastrozole (SoFEA) — will determine whether we can translate that into the clinical setting.
EFECT and SoFEA trials
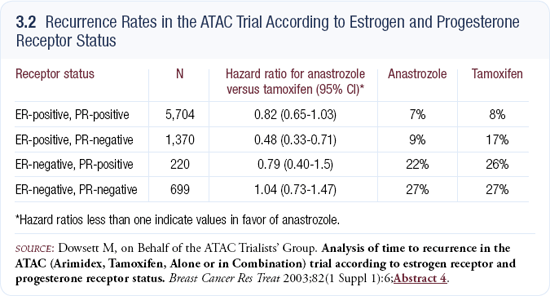
EFECT is an American and European study that will randomly assign patients who have failed therapy with a nonsteroidal aromatase inhibitor to fulvestrant or exemestane. Our own study, SoFEA (3.3), is slightly different from EFECT because it is based on the observation that the addition of small amounts of estrogen to cells that have been estrogen-deprived for a long time reduces the effectiveness of fulvestrant.
That scenario equates to the withdrawal of a nonsteroidal aromatase inhibitor and the addition of fulvestrant. Hence, the third arm of our trial includes a nonsteroidal aromatase inhibitor and fulvestrant. The SoFEA trial will randomly assign 750 patients who have failed therapy with a nonsteroidal aromatase inhibitor to exemestane, fulvestrant alone or fulvestrant plus anastrozole.
I predict fulvestrant alone will probably be better than exemestane, and fulvestrant plus anastrozole will be better than fulvestrant alone. In that particular study, we are using a loading-dose schedule for fulvestrant — 500 mg initially, followed two weeks later with another 250 mg, and then monthly injections.
Since fulvestrant has a long half-life of about 40 days, it takes a long time to reach steady state levels. This strategy allows the fulvestrant levels to reach steady state and the drug to be effective more quickly.
Phenotypic changes induced by tamoxifen therapy
We constructed a tissue microarray from the tumors of 39 patients who became resistant to adjuvant tamoxifen. We had pretreatment samples taken at excision and samples taken at the time of relapse on adjuvant tamoxifen.
Initially, 29 patients had ER-positive disease. At the time of relapse, five of those patients had ER-negative disease and the other 24 had ER-positive disease. Hence, different mechanisms might be operative in tamoxifen resistance.
More surprising, three patients who initially had ER-positive, HER2-negative disease had HER2-positive disease at the time of relapse. Of the 29 patients who initially had ER-positive disease, seven had a change in their phenotype.
If we had treated those seven patients based on their pretreatment specimens, we would have either treated them with endocrine therapy or denied them trastuzumab inappropriately. These patients accounted for 24 percent of the total population, so a greater focus should be placed on trying to obtain biopsy specimens from patients at the time of relapse.
I should add one cautionary remark: most patients had local relapses. We need to confirm a new primary wasn’t misdiagnosed. We’re currently doing molecular analyses — comparative genomic hybridization between the pretreatment and the relapse specimens — to confirm that those patients had relapses and not new tumors.
The majority of the data comparing the HER2 status in primary and metastatic disease has evaluated lymph nodes, which is not quite comparable to our data. A study from a Belgian group of 106 patients found an approximately five or six percent difference in HER2 status, which is not much different from our finding in 29 patients. If the metastases become HER2-positive, we ought to know that to consider using trastuzumab.
HERA trial of adjuvant trastuzumab
The HERA trial (3.4) is a relatively pragmatic study. Patients initially receive an approved adjuvant chemotherapy regimen, and then they are randomly assigned to trastuzumab monotherapy for either one or two years or no trastuzumab. It’s my responsibility and that of Brian Leyland-Jones, who co-chairs the Trans-HERA Committee, to collect the tumor blocks from that trial and perform biomarker analyses.
Select publications
 |
Dr Dowsett is a Professor of Biochemical Endocrinology and Head of the Academic Department of Biochemistry at Royal Marsden Hospital in London, England. |
|
