Monica Morrow, MD |
EDITED COMMENTS |
 Current status of sentinel lymph node biopsy for staging breast cancer Current status of sentinel lymph node biopsy for staging breast cancer
We now have clear data that sentinel lymph node biopsy (SLNB) is the staging procedure of choice for clinically node-negative breast cancer. Previously no long-term follow-up data was available, but now over 4,000 cases have been published with a mean follow-up of at least two years, which is when most axillary recurrences occur.
The incidence of isolated axillary failure is one tenth of one percent, which is very low. Additionally, we now have two randomized trials evaluating the incidence of nodal positivity in women staged by sentinel node biopsy versus axillary dissection (Mansel 2004, Veronesi 2003).
The known false-negative rate of SLNB has always been a concern to some people. Even in the best of hands, a false-negative rate of three to five percent occurs — closer to 10 percent in multi-institutional studies. From these randomized trials in patients who were stratified by tumor size, the likelihood of finding a positive node is the same in the SLNB group as those who underwent axillary dissection, which suggests those are false negatives, probably in axillary dissection as well. I suspect these are the failure of the pathologist to identify the nodal metastases as opposed to failure to remove the proper node as seen in SLNB.
Sentinel node biopsy provides staging accuracy equivalent to axillary dissection, and the morbidity is clearly less — not only the immediate postoperative morbidity but also two years later in measurable differences in pain, paresthesia, arm motion and lymphedema. Additionally, we now know long-term local tumor control is good.
NSABP and American College of Surgeons trials of SLNB
The NSABP study included women with clinically node-negative disease who underwent a sentinel node biopsy. If the sentinel node was positive, they underwent an axillary dissection. If the sentinel node was negative, they were randomly assigned to undergo complete axillary dissection or observation. Many people, including myself, consider that an unusual question to ask. Why would removing negative axillary lymph nodes contribute to survival, given that the NSABP asked and answered that question a number of years ago in their B-04 trial?
You could say their trial is a complicated quality control check. That study has closed to accrual but the data have not yet been presented. The other important question the study is addressing is the prognostic significance of “micrometastases” — immunohistochemically detected cells in the sentinel node.
The American College of Surgeons (ACOS) is conducting two major studies. The first is simply an observational study in patients who had clinically nodenegative disease and were sentinel node-negative by H&E. They had no further axillary treatment, but immunohistochemistry was performed for prognostic purposes with a companion bone marrow study to evaluate “ultrastaging.” Some impressive data sets from Europe suggest that bone marrow micrometastases are independent prognostic factors, and this study attempts to validate that concept.
The more important study from the ACOS is the Z-11 trial in which patients with an H&E-positive sentinel node are randomly assigned to complete axillary dissection or axillary observation. The main aim of the trial is to definitively answer the question: Does axillary dissection offer a survival benefit?
SLNB and IHC-detected micrometastases
In our practice we ask the pathologist not to routinely perform IHC on the sentinel node because we’ve concluded that we don’t know what it means based on the available data, which continues to be confusing. A prospective study from the John Wayne Cancer Institute separated patients with standard H&Edetected macrometastases, patients with micrometastases less than two millimeters detected by H&E, patients with IHC-positive disease and patients with truly node-negative disease.
At a follow-up of approximately four and a half years, the patients with macrometastases had a significantly lower disease-free survival than anticipated. The disease-free survival curves for the other arms were completely overlapping.
The Italians discovered a disease-free survival difference in the patients with IHC-detected micrometastases. These findings illustrate that in this complicated arena — where different adjuvant therapies and the limitations of nonprospectively designed studies will influence the outcome — we have to wait to know for certain the meaning of IHC-detected micrometastases from the NSABP and American College of Surgeons trials.
From a practical standpoint, if a patient has multiple sentinel nodes with IHCpositive cells, it’s a matter of tumor burden, and we would treat her as if she had node-positive disease. The problem occurs when you see a couple of cells in a subcapsule or sinus, and you don’t know if they have the potential to grow or are just intransigent.
We don’t really know what to do with those patients. If the patient has ER-positive disease, administering endocrine therapy is perfectly satisfactory. The clinical dilemma arises when you identify the patient as having node-positive disease, because then you would administer chemotherapy and endocrine therapy.
SLNB in the neoadjuvant setting
Initially, based on a number of small neoadjuvant studies in which the accuracy of the sentinel node biopsy was highly variable, my preference was to perform the sentinel node biopsy prior to the initiation of neoadjuvant therapy, so that it wasn’t confounded in any way. If the sentinel node was positive, I would perform the axillary dissection later at the time of definitive breast surgery.
The SLNB data from the NSABP neoadjuvant trial made me much more comfortable performing the sentinel node biopsy after delivery of chemotherapy, recognizing this was not part of the trial design, but nonetheless, is a 427-patient data set that shows the same kind of accuracy of staging as seen in all multi-institution sentinel node trials.
From a practical point of view, in a patient who has received neoadjuvant therapy the decision is made whether to administer more treatment postoperatively based on the total extent of tumor response. In these cases, the lymph node is not nearly as decisive as it might be in a conventional patient who’s having pure postoperative therapy.
The one argument in favor of continuing to perform pretreatment SLNB comes from our radiation oncology colleagues who have a great deal of difficulty figuring out what volume to treat. Those who are in favor of more aggressive irradiation in patients with positive nodes claim that knowing the nodal status prior to chemotherapy helps them, for example, to decide whether or not to treat supraclavicular or nodal fields.
MD Anderson study of neoadjuvant trastuzumab and chemotherapy
The pathologic complete response rate in the MD Anderson neoadjuvant trastuzumab trial (Buzdar 2004) of about 65 percent was unlike anything we’ve seen in any other trial of combination chemotherapy. Given that tumors overexpressing HER2 are often large and more rapidly growing, those findings have the potential to benefit many women.
My medical oncology colleagues expressed a lot of concerns about the small number of patients reported and potential toxicity in that setting. If I could see similar responses repeated in another data set, I would consider neoadjuvant trastuzumab and chemotherapy a worthwhile option for patients who met their criteria.
I would be uncomfortable with adjuvant trastuzumab in a nonprotocol setting; however, the line between inflammatory and metastatic breast cancer is not significant. Certainly, in a patient who receives conventional up-front chemotherapy and does not achieve a complete response to their inflammatory disease, and you can’t operate on them and can’t achieve local control, the addition of trastuzumab is perfectly reasonable.
Use of aromatase inhibitors in the adjuvant setting
Whether surgeons prescribe adjuvant aromatase inhibitors will depend on their level of comfort with the literature, counseling patients and dealing with any potential side effects. Adjuvant endocrine therapy has become much more complex in terms of the various options and what’s known and unknown.
For postmenopausal patients who have been on adjuvant tamoxifen for two to three years, I refer them to the oncologist to determine if they should be switched to an aromatase inhibitor, unless they have very low-risk disease. In patients with tumors less than two centimeters who have node-negative disease and are tolerating tamoxifen, my threshold to switch to an aromatase inhibitor is higher. Any patient who has a higher risk of relapse deserves to have a discussion about switching to an aromatase inhibitor (Boccardo 2003, Coombes 2004) as we do in women who have completed five years of tamoxifen (Goss 2003).
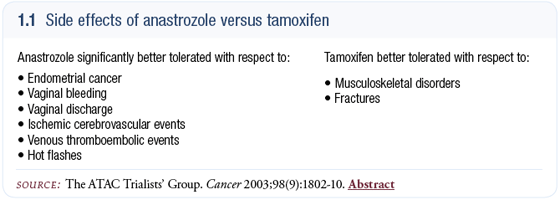
The aromatase inhibitors cause some non-life-threatening side effects — such as musculoskeletal aches and pains — more than I expected based on the clinical trial data (1.1). On the other hand, I have been favorably impressed by the clear difference in vasomotor symptoms encountered with the aromatase inhibitors and the decreased rate of thrombosis and endometrial cancer compared to tamoxifen.
Select publications
 |
Dr Morrow is Chairman of the Department of Surgical Oncology and G Willing Pepper Chair in Cancer Research at Fox Chase Cancer Center in Philadelphia, Pennsylvania. |
|

