Harold J Burstein, MD, PhD |
EDITED COMMENTS |
 Incorporation of aromatase inhibitors in the adjuvant setting Incorporation of aromatase inhibitors in the adjuvant setting
Recently, we have received data on adjuvant therapy from the three largest treatment trials in the history of medical oncology — ATAC (Baum, 2003) with 9,000 patients and the CAN-NCIC-MA17 (Goss, 2003) and EU-20149 (Coombes, 2004) trials with approximately 5,000 patients each.
The ATAC trial examined front-line therapy comparing tamoxifen, anastrozole and the combination. The MA17 trial evaluated letrozole versus placebo after five years of adjuvant tamoxifen, and the EU-20149 study evaluated continued tamoxifen versus switching to exemestane after two to three years of adjuvant tamoxifen. A small but important Italian trial evaluated anastrozole after two to three years of tamoxifen.
While these trials provide a tremendous reservoir of data, they each examined a different sequence and utilized a different aromatase inhibitor. Nonetheless, they all showed a benefit with aromatase inhibitors, and it is currently believed that postmenopausal patients diagnosed early with hormone receptor-positive breast cancer probably should receive one of these agents at some point for optimal adjuvant therapy.
Still, we don’t really know the ideal time to begin an aromatase inhibitor or how to sequence these agents around tamoxifen. Nor do we know how long patients should take an aromatase inhibitor or which one is the most beneficial.
Toxicities of adjuvant endocrine therapies
In addition to efficacy, the other important data we can glean from the large adjuvant endocrine trials is the collective toxicity experience. All three studies demonstrated a greater risk of osteoporosis, osteoporotic fractures and musculoskeletal problems with aromatase inhibitors compared to tamoxifen.
A significant difference does not appear to be evident in the rates of bone loss, sexual dysfunction or musculoskeletal events between the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitors anastrozole and letrozole. On the other hand, tamoxifen is associated with an increased risk of endometrial cancer and deep vein thrombosis, which are rare but worrisome side effects.
Bisphosphonates were not utilized in the adjuvant trials and they may reduce the incidence of bone loss; however, we have no substantial data on how best to treat osteopenia or osteoporosis in postmenopausal women taking aromatase inhibitors.
Trials examining this issue are underway and I suspect they will come up with effective strategies to deal with this toxicity. We also need to emphasize other strategies that can reduce bone loss, including weight-bearing exercises, treatment for other medical problems like thyroid conditions, calcium and vitamin D supplementation and smoking cessation.
Sequencing of endocrine therapy in the adjuvant setting
When deciding which endocrine therapy to utilize, I discuss the data with the patient and contrast the side effects of the different agents. If no patient-specific reason exists to avoid a drug — such as a history of deep vein thrombosis or severe osteoporosis — I generally begin with tamoxifen with the intent to switch to exemestane after two to three years, or I use anastrozole up front. Based on the subset analysis from ATAC, in a patient with ER-positive, PR-negative disease, I’m inclined to use anastrozole initially.
For the postmenopausal patient who has been doing well on tamoxifen for two or three years, I generally switch them to exemestane based on the EU-20149 data. Similarly, for patients who are finishing five years of tamoxifen, I frequently offer them letrozole based on MA17. I find the MA17 data particularly compelling for the patients at higher risk, because of the ongoing residual risk for recurrence years after the initial diagnosis.
In women with low-risk disease, the benefit is less and if a patient with a history of a tiny, node-negative breast tumor has been counting the days until she can quit her tamoxifen because she suffers from hot flashes, I’m less certain continued therapy is best for the patient. However, for most women at average risk, I strongly encourage letrozole after five years of tamoxifen.
After a patient completes five years of adjuvant tamoxifen, we don’t know whether she benefits from further therapy begun six months, one year or three years later. MA17 included women who had finished tamoxifen within six months, and I believe it’s reasonable to extrapolate the data to women who have completed tamoxifen within the past year or so.
However, for a woman who is recurrence-free three years after completing tamoxifen, the risk of recurrence has declined further, and it’s difficult to know how the data relate to her. I would only offer that woman further therapy if she was particularly motivated to continue adjuvant therapy.
Adjuvant endocrine therapy and HER2 status
Within the next year or two we will have data from the adjuvant trastuzumab trials, and I expect these will be positive. If this happens, the 20 percent of patients with HER2-positive breast cancer will be treated differently and removed from the pool of patients with ER-positive breast cancer, and the prognosis for the remaining 80 percent will improve because we removed the poor-prognosis group.
For those remaining women, hormone therapy will be even more important, because it’s the patients with HER2-positive disease that historically have been more resistant to endocrine treatment.
HER2-positive breast cancer is probably less sensitive to the effects of tamoxifen than HER2-negative disease for a couple of reasons. One is the cross talk between the HER2 protein and estrogen- and progesterone-receptor protein pathways. The other is that in tumors that express both ER and HER2, the quantitative level of expression of ER is much less than women with ER-positive, HER2-negative tumors.
Algorithm for HER2 testing
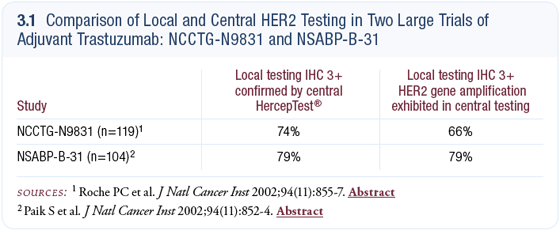
HER2 testing should be ordered up front routinely for all patients. At our institution, we perform IHC initially and if the results are 3+, we consider it positive; zero or 1+ is considered negative, and 2+ results are sent for FISH testing. All clinicians should know the ability of their pathology department to perform these tests accurately. The literature suggests that smaller laboratories and pathology centers that perform relatively few tests tend to have fairly substantial rates of false-positive and false-negative findings (3.1).
Interestingly, that data has spread quickly throughout the pathology community, and I believe tremendous quality improvements have been made. The results today are more reliable than even just a few years ago. Physicians should ask their pathologist what the concordance is between their local testing and a referral lab and if they can’t tell you, then you need to have it sent out to a referral lab that can.

MD Anderson preoperative trial of trastuzumab and chemotherapy
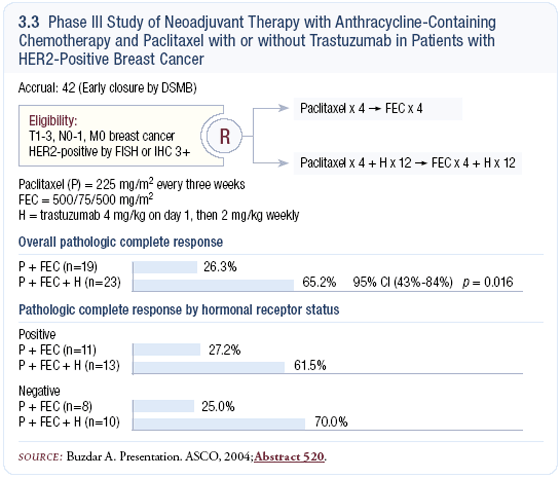
These patients had HER2-positive Stage II or III breast cancer and were randomly assigned to preoperative chemotherapy with or without trastuzumab (3.3). The study was designed to accrue between 150 and 200 patients, but it was closed after treating approximately 40 patients because of a robust difference in the pathologic complete response rates.
While women receiving anthracycline-containing chemotherapy alone had a pathologic complete response rate of approximately 20 percent, those who received chemotherapy plus trastuzumab had a pathologic complete response rate over 60 percent. They have stopped the randomization; however, they have continued treatment on the trastuzumab arm to acquire greater safety experience and to validate the findings.
Despite the early closure of this randomized trial, its findings are provocative. It demonstrates that trastuzumab is active in the preoperative setting and results in a complete remission in a much larger percentage of patients. If we think about the number of papers reporting on various regimens of preoperative chemotherapy, never stratified by HER2, they’ve always shown pathologic complete response rates of 15 to 20 percent, especially in hormone receptornegative tumors.
This literature just became irrelevant because we now know that we can triple the pathologic complete response rate in HER2-positive tumors by adding trastuzumab. It’s very exciting to be in clinical research right now, because we’re learning that when we target these subsets of breast cancer with biologically focused therapies, we can radically change everything we know about treating this disease.
Select publications
 |
Dr Burstein is an Assistant Professor of Medicine at Harvard Medical School and the Breast Oncology Center at the Dana-Farber Cancer Institute in Boston, Massachusetts. |
|

