J Michael Dixon, BSc, MBChB, MD, FRCS, FRCSEd |
EDITED COMMENTS |
 IMPACT neoadjuvant trial: A comparison of anastrozole, tamoxifen and the combination IMPACT neoadjuvant trial: A comparison of anastrozole, tamoxifen and the combination
Initially, the main objective of the IMPACT trial was biologic — to determine how cancer changes after treatment with anastrozole, tamoxifen or the two combined. In fact, the initial plan was to solely focus on biologic endpoints, and rigorous clinical evaluation was added in later.
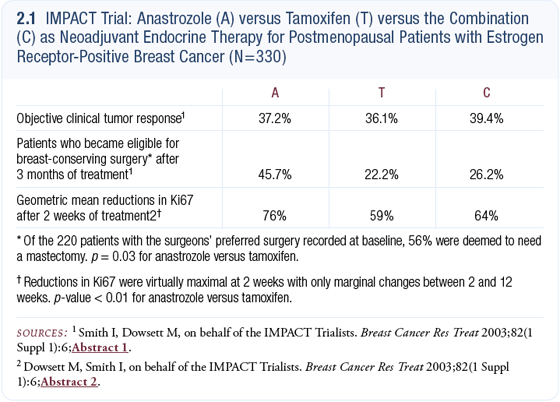
The trial included patients regardless of their tumor size, and many of the patients had small tumors. It is clinically difficult to detect responses in small tumors; therefore, I was not surprised that the IMPACT trial didn’t demonstrate major clinical differences (Smith 2003). A difference was found in the patients with larger tumors; they were more likely to have breast-conserving surgery if they were treated with neoadjuvant anastrozole (2.1) — about twice that rate.
Those findings support the results from the neoadjuvant letrozole study, which demonstrated that a larger percentage of patients treated with letrozole had breast-conserving surgery than those receiving tamoxifen (Ellis 2001).
I believe the IMPACT trial demonstrates the poor utility of clinical response as an endpoint in neoadjuvant trials. In many respects, reduction in tumor volume is more valuable. If reduction in tumor volume had been evaluated for the patients in the IMPACT trial, I suspect the trial would have demonstrated that anastrozole was superior, as evidenced by the fact that more patients with larger tumors had breast-conserving surgery.
For surgeons who want to shrink larger tumors and be able to perform breastconserving surgery, it’s not just response but the degree of response that is important. In our neoadjuvant studies, the reduction in tumor volume was much better with all of the aromatase inhibitors (including anastrozole) compared to tamoxifen.
IMPACT neoadjuvant trial: Biologic endpoints
We collected tissue at baseline, 14 days and three months from the patients on the IMPACT study. We’re currently completing the microarray assays on those samples and have sufficient samples from the different treatment groups to determine which genes are switched on or off and whether the tumors become less aggressive at the end of the treatment period.
In a previous uncontrolled study, we found that anastrozole and letrozole reduced proliferation after 14 days of treatment. In the IMPACT trial, tamoxifen alone and the combination were identical after two weeks in terms of their ability to reduce proliferation. On the other hand, anastrozole was significantly better at reducing proliferation than either of the other two treatments (2.1) (Dowsett 2003a).
I don’t suppose the biologic endpoints would have been believable if the ATAC adjuvant trial hadn’t demonstrated that anastrozole was superior to tamoxifen and the combination (Baum 2003). For those of us who believe in these biologic models, it’s great that the biologic model was validated in a large adjuvant study. I hope this will allow us to use some of these biologic markers as endpoints in future trials.
ATAC adjuvant trial: Benefits according to estrogen- and progesterone-receptor status
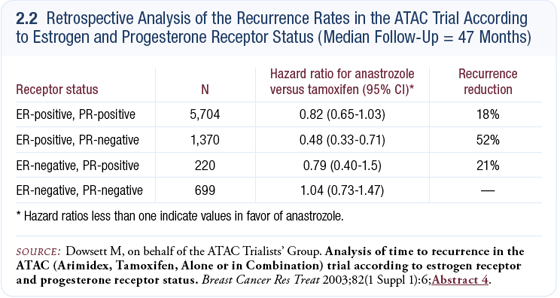
In the ATAC adjuvant trial, we saw differences between anastrozole and tamoxifen based on their estrogen receptor (ER) and progesterone receptor (PR) expression. In the women with ER-positive and PR-negative tumors, the difference was great and was quite surprising — about a 50% reduction in recurrence rate in anastrozole compared to tamoxifen (2.2) (Dowsett 2003b).
Some data suggest that women with PR-negative disease are more likely to also have HER2-positive disease, whereas other data indicate that this is true for younger women, but not for older women. The great thing about the trans-ATAC trial, being run by Mitch Dowsett, is that they’re collecting all of the tumor blocks, and we will soon learn the answer. It is our belief that the aromatase inhibitors have a greater effect than tamoxifen in patients with HER2-positive tumors.
Sequential adjuvant hormonal therapy in postmenopausal women
It’s not surprising that switching from adjuvant tamoxifen to a more powerful aromatase inhibitor improves outcome. In an era in which most patients will initially receive an adjuvant aromatase inhibitor, the issues include the duration of therapy with the aromatase inhibitor and whether those patients should at some point be switched to tamoxifen.
I believe the optimal strategy will be to start out with an adjuvant aromatase inhibitor. If adjuvant therapy is initiated with tamoxifen, many patients will have a recurrence before the best drug is used. One of the things we’ve learned in the treatment of cancer is to use the best agent first.
In terms of how long after completing five years of adjuvant tamoxifen an aromatase inhibitor should be initiated, the only relevant data are from a trial in patients who were treated with delayed adjuvant tamoxifen. That trial found that those women, even if they received adjuvant tamoxifen up to five years later, had an improvement in survival compared to women who never received adjuvant tamoxifen (Delozier 2000).
Therefore, I suspect that women who have been off of adjuvant tamoxifen for some time and are treated with an aromatase inhibitor will benefit compared to women who do not receive an aromatase inhibitor. All of our decisions are based on risk-to-benefit ratios. The benefits must be sufficient to consider treating a patient with drugs that have inherent risks.
IBIS-II prevention trial in postmenopausal women
The IBIS-II prevention trial will compare the aromatase inhibitor anastrozole to placebo. We’ll all be surprised if anastrozole does not reduce the incidence of breast cancer. Although we’re currently fixated on the bone effects associated with the aromatase inhibitors, I believe we will find that with the new powerful bisphosphonates, the bone effects will not be a long-term problem.
One of the strengths of the IBIS-II prevention trial is that it will help identify women in whom we need to do bone scans and those in whom we need to use bisphosphonates. In the separate bone subprotocol of the IBIS-II prevention trial, women with a high baseline bone mineral density (BMD) won’t receive a bisphosphonate, women with a low baseline BMD will automatically receive a bisphosphonate, and women with a baseline BMD in the midrange will be randomly assigned to a bisphosphonate or placebo.
Select publications
 |
Dr Dixon is a Consultant Surgeon and Senior Lecturer in Surgery in the Edinburgh Breast Unit at Western General Hospital in Edinburgh, United Kingdom. |
|

