|
You are here: Home: BCU 3|2001: Section 8

Section 8
Capecitabine for Metastatic Disease
I think we underestimate
the efficacy of capecitabine on the basis of the published percentages
of response. Some of the responses that I’m observing in the
clinic are very long, and somehow that doesn’t come across
in the papers. Also, there’s another 20-30 percent of patients
who achieve very high quality stable disease — akin to what
we observe with endocrine therapy. So, I think capecitabine in second-
or third-line therapy is a substantial contribution to palliative
treatment. My group uses capecitabine a lot, and sometimes —
in patients for whom a high quality of life is the first priority
— we might use single-agent capecitabine in first-line therapy
for metastatic breast cancer. There are now planned trials of capecitabine
in the adjuvant setting, so we will go through several years of
exploring capecitabine alone and in combination in various settings.
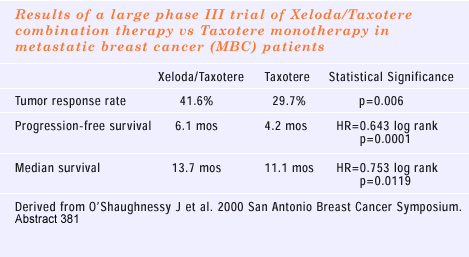
The recent preclinical data showing an interaction with Taxotere
is also very interesting. It has been demonstrated that capecitabine
plus docetaxel has a higher response rate and even a longer survival
than docetaxel alone. What would make that a very compelling combination
is if we answer the second question, which is: Does the sequential
use of these two agents provide inferior results to the simultaneous
combination?
—Gabriel
Hortobagyi, MD
In the second-line
setting, I believe that sequential utilization of agents results
in optimal palliation. After patients progress on a taxane and anthracycline,
the drug that I use and prefer is capecitabine, because it has established
antitumor activity and it is an oral agent. In studies of patients
who were anthracycline- and taxane-resistant, a little more than
half of the patients had clinical benefit and control of their disease.
Another important aspect of this drug is that it is not myelosuppressive.
Some of these patients have gone through high-dose or intensive
chemotherapy, and even though their blood counts are normal, they
can’t tolerate another myelosuppressive agent, even at a standard
dose. The package insert for capecitabine calls for 2,500 mg/m2,
but in a substantial number of patients, the dose has to be reduced.Outside
the context of clinical trial, I usually start with 2,000 mg/m2,
and that dose then may have to be fine-tuned.
—Aman
Buzdar, MD
SELECT
PUBLICATIONS
|
Blum JL
et al. Multicenter phase II study of capecitabine in paclitaxel-
refractory metastatic breast cancer. J Clin Oncol
1999;17(2):485-93. Abstract
Blum JL
et al. A multicenter phase II trial of Xeloda TM
(capecitabine) in taxane-refractory metastatic breast cancer.
Proc ASCO 1999; Abstract
403.
Blum JL.
The role of capecitabine, an oral, enzymatically activated
fluoropyrimidine, in the treatment of metastatic breast cancer.
Oncologist 2001;6(1):56-64. Abstract
Budman
DR et al. Preliminary studies of a novel oral fluoropyrimidine
carbamate: Capecitabine. J Clin Oncol 1998;16:1795-1802.
Abstract
Gradishar
WJ. Clinical status of capecitabine in the treatment of
breast cancer. Oncology (Huntingt)2001;15(1 Suppl
2):69-71;discussion 72. Abstract
Mackean
M et al. Phase I and pharmacologic study of intermittent
twice-daily oral therapy with capecitabine in patients with
advanced and/or metastatic cancer. J Clin Oncol 1998;16:2977-2985.
Abstract
Michaud
LB et al. Improved therapeutic index with lower dose capecitabine
in metastatic breast cancer (MBC) patients (Pts). Proc
ASCO 2000; Abstract
402.
Thuss-Patience
PC et al. Capecitabine: A new standard in metastatic breast
cancer recurring after anthracycline and taxane-containing
chemotherapy? Results of a multicenter phase II trial.
Proc ASCO 2001; Abstract
2012.
|
|
|
