| You are here: Home: BCU 3|2001: Section 1

Section 1
Neoadjuvant
Endocrine Therapy
RESEARCH
BACKGROUND
We began studying
neoadjuvant systemic therapy about 15 years ago, and one major reason
was that we thought accurate models of drug action could be derived
by taking sequential samples of tumors during treatment.What’s
clear is that you can obtain a very good idea of what’s happening
within the tumors by looking at biological markers rather than just
clinical endpoints.
We take biopsies after 10 to 14 days of treatment and examine effects
on proliferation, cell death and a variety of genetic markers to
determine if we can predict early on whether a patient is going
to receive benefit from therapy.This then becomes a potential method
to individualize treatment. We found that the median time to reduce
tumor volume is shorter with chemotherapy than with endocrine therapy.
However, the same order of response is seen with endocrine therapy
— you just have to wait a bit longer.
—
J Michael Dixon, MD
NEOADJUVANT
AROMATASE INHIBITORS
The time to
response and volume of tumor reduction seems to be greater with
aromatase inhibitors than with tamoxifen, and quite marked tumor
reductions are often seen within four weeks. Defining responses
as greater than a 50% reduction in tumor volume, we have observed
response rates of 80% to 90% with the aromatase inhibitors, which
is quite impressive.
With one milligram
of anastrozole, after three months, we saw an 80% overall reduction
in tumor volume.These agents suppress circulating estrogen production
and intratumoral estrogen production. After a few months of therapy,
the levels of intratumoral estrogens are incredibly small.
—
J Michael Dixon, MD
Examples
of responses to neoadjuvant anastrozole as assessed by changes in
tumor volume measured mammographically.
Sahni
S et al. Evaluation of local and systemic disease control following
breast conserving surgery after neoadjuvant anastrozole treatment.
Poster 2001 Miami Breast Cancer Conference. Full
Text.

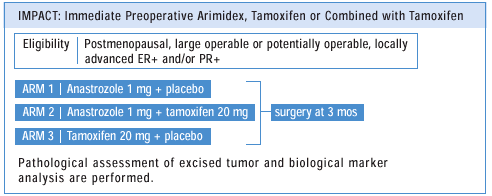
NEOADJUVANT
TRIAL OF ARIMIMIDEX® (anastrozole ) VS TAMOXIFEN VS THE COMBINATION
We
are currently studying in a neoadjuvant setting the same therapies
being evaluated in the ATAC adjuvant trial.From our prior work,
we know that anastrozole and tamoxifen have different biological
effects on tumors. Anastrozole switches off proliferation. If you
look at the tumor before starting anastrozole and then three months
later, in about half of the patients the actual histologic grade
changes — it downgrades the tumor. So, if it’s a grade
3, it ’ll go to 2, and grade 2 will go to 1. But the reason
it downgrades is because proliferation is turned off.
If
you look at tamoxifen, about half of the patients will also downgrade,
but that downgrade is completely different. What you see is an increase
in glandular differentiation. Tamoxifen also tends to induce the
progesterone receptor, while anastrozole switches the PR off completely.
These observations fit with the fact that different clinical activities
have been seen with aromatase inhibitors, which are actually more
effective than tamoxifen. So, we’re starting to understand
that there’s not one pathway for endocrine therapies. And,
of course, if they act by different mechanisms, then combinations
— like in ATAC — could be more effective than one agent
alone.
—
J Michael Dixon, MD
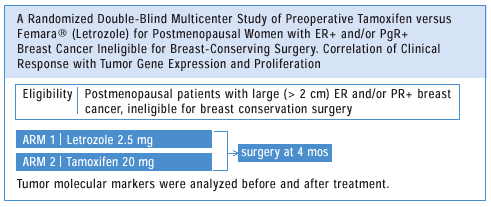
OTHER
TRIALS OF NEOADJUVANT AROMATASE INHIBITORS

Proact
Protocol
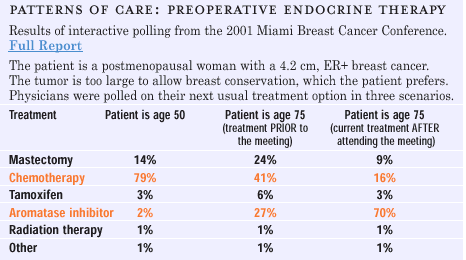
CLINICAL
USE OF NEOADJUVANT ENDOCRINE THERAPY
What
has always impressed me about neoadjuvant endocrine therapy is that
if patients are selected properly, a similar change in tumor volume
can be achieved as with chemotherapy. It requires a slightly longer
time period, but there are far fewer side effects. Our quality of
life studies show that patients don ’t seem to mind taking
adjuvant endocrine therapy for a few months, because there are very
few side effects. We have a group of elderly patients who avoid
medical care, because they don ’t want to undergo extensive
surgery. Many of the tumors in these women are quite large and estrogen
receptor-rich. After neoadjuvant endocrine therapy, mastectomy is
usually not required, and patients can have much less deforming
surgery.
Another
important issue is that in women over age 70, there ’s a one
percent mortality rate in most published series of mastectomy and
axillary dissection, because the surgery can be quite stressful.
It ’s a longer operation with the potential for more complications.
So, by doing less surgery, you have less chance of patients dying
of vascular events or developing complications such as hematomas.
The women we ’ve treated with neoadjuvant endocrine therapy
have been the happiest group of patients in our practice.
—
J Michael Dixon, MD
SELECT
PUBLICATIONS
|
Aapro
MS. Neoadjuvant therapy in breast cancer:Can we define
its role? Oncologist 2001;6 Suppl 3:36-9. Abstract
Cheung KL et al. Preoperative endocrine therapy for breast
cancer.
Endocrine-Related Cancer 1999;7(3):131-141.
Full
Text
Dixon
JM et al.The effects of neoadjuvant anastrozole (Arimidex)on
tumor volume in postmenopausal women with breast cancer:A
randomized,double-blind,single-center study. Clin Cancer
Res 2000;6(6):2229-35. Abstract
Dixon
JM et al. Lessons from the use of aromatase inhibitors
in the neoadjuvant setting. Endocrine-Related Cancer
1999;6(2):227-230. Full
Text
Ellis MJ. Preoperative endocrine therapy for older women
with breast cancer: Renewed interest in an old idea. Cancer
Control 2000;7(6):557. Full
Text
Geisler
J et al. Influence of neoadjuvant anastrozole (Arimidex)on
intratumoral estrogen levels and proliferation markers in
patients with locally advanced breast cancer.
Clin Cancer Res 2001;7(5):1230-6. Abstract
Gianni
L. Adjuvant and neoadjuvant treatment of breast cancer.
Semin Oncol 2001;28(1):13-29. Abstract
Hoff PM
et al. Combined modality treatment of locally advanced
breast carcinoma in elderly patients or patients with severe
comorbid conditions using tamoxifen as the primary therapy.
Cancer 2000;88(9):2054-60. Abstract
Makris
A et al. Reduction in angiogenesis after neoadjuvant chemoendocrine
therapy in patients with operable breast carcinoma. Cancer
1999;85(9):1996-2000. Abstract
Pujol
P et al. Neoadjuvant tamoxifen for operable breast cancer:A
need for phase III studies? Cancer Detect Prev
2000;24(5):445-51. Abstract
Smith
IC, Miller ID. Issues involved in research into the neoadjuvant
treatment of breast cancer. Anticancer Drugs 2001;12
Suppl 1:S25-9. Abstract
Smith
IC et al. Current and potential chemotherapeutic agents
used for induction chemotherapy in the treatment of breast
cancer. Curr Pharm Des 2000;6(3):327-43. Abstract
Terashima M et al. Breast-conservation treatment for patients
with ductal carcinoma in situ. Oncol Rep 2000;7(6):1247-52.
Abstract
|
|
