|
You are here: Home: BCU 3|2001: Section 3

Section
3
Taxanes in the Adjuvant
and Metastatic Setting
NIH
CONSENSUS RECOMMENDATIONS
I understand
the NCI consensus statement about adjuvant taxanes, but that panel
did not have expertise in breast cancer — it was a group of
people being asked to make recommendations by jury. Taxol® (paclitaxel)
is approved for adjuvant therapy of high-risk breast cancer patients,
and not only did the Intergroup study demonstrate benefit, but our
smaller MD Anderson study also showed a reduction in risk of recurrence,
which was totally independent of receptor status. Also, about one-third
of our patients received Taxol in the neoadjuvant setting, where
the response could be assessed directly, and there was no difference
in response in ER-positive or ER-negative patients. Our study had
very few deaths, so we can’t
talk about survival. Women need to be active participants in the
decision-making process, but I believe that it is appropriate to
offer Taxol to a woman who is at a high risk, i.e., has positive
nodes. Even for large node-negative tumors I tell patients that
FAC chemotherapy will reduce the risk of recurrence, and if we add
a taxane, it will further reduce that risk. In terms of side effects,
you’re talking about essentially 12 weeks of additional therapy,
which substantially reduces the risk of recurrence, and as the data
mature, I am very optimistic that it will reduce the risk of death.
—Aman
Buzdar, MD
| Adjuvant
Taxanes: Another Viewpoint |
|
Editor’s
Note: The accompanying audio program includes comments from
Dr Aman Buzdar supporting the option of adjuvant Taxol for
both node-positive and high-risk node-negative patients. Dr
Buzdar believes that current available trial data demonstrate
an advantage in disease-free survival regardless of estrogen
receptor status.Other research leaders interviewed for Breast
Cancer Update have been more conservative in their interpretation
of the available data.In Program 1, 2001, Richard Elledge,
MD, discussed his perspective on this controversial issue.
Dr Elledge:
The initial Intergroup
0148 trial was analyzed at a point that was very early
relative to when we usually analyze adjuvant therapy trials,
which made some people a little bit less confident of the
data — though it was a large, well-done study. Now we’re
seeing that there’s apparently less of a benefit with
further follow-up. On top of that, the NSABP
B-28 trial — which was more or less the same trial
design as the Intergroup trial — has been analyzed with
approximately three years of follow-up, and there’s no
significant benefit with the addition of Taxol. So once again,
in breast cancer clinical research, our intuition and hopes
shouldn’t run away with what the data say, and I believe
we can serve patients best if we can stick close to the data.
Neil Love, MD: How have you approached
the use of adjuvant taxanes in the nonprotocol setting?
Dr Elledge: I was always uncomfortable with the Intergroup
trial, because it was only one study, which was analyzed early.
And, while it was a subset analysis, even in that trial there
was no significant benefit in ER-positive patients. Because
of that, outside of a study, I am not giving taxanes to ER-positive
patients unless they have a lot of positive nodes. For ER-negative
patients, again, if they have many positive nodes, I usually
recommend including a taxane.
Dr Love: It sounds like you think there may be an advantage
for
adding a taxane.
Dr Elledge: I’m hoping that there is, but I’m growing
more skeptical. It has been suggested that this may end up
being like the anthracycline story, in that we went back and
forth for years trying to determine whether the addition of
an anthracycline was really beneficial in the adjuvant setting.
And in the end, data now support that there is a very small
difference to adding an anthracycline. It’s unfortunate
that these differences are so small. When you look at the
addition of anthracyclines in the node-negative population
in the Intergroup trial, we’re talking about differences
of one and two percent increments in survival, and it may
be that that’s the way the taxanes will pan out. That
certainly would be a disappointment.
|
SCHEDULE,
TIMING AND CHOICE THERAPY
Our surgeons have agreed that we will give all systemic therapy
up front and then follow that with local therapy — except for
patients with tumors that are one centimeter or less, where the
woman is a candidate for breast preservation. For other patients,
we believe that if the patient needs chemotherapy, it’s better
to do it preoperatively. It’s a very easy way to find out whether
the tumor is going to respond — it’s an in vivo sensitivity
assay. If the tumor volume is substantially reduced, the patient
has a much better chance of remaining disease-free compared to a
woman whose tumor does not change, where one should consider switching
to another regimen.
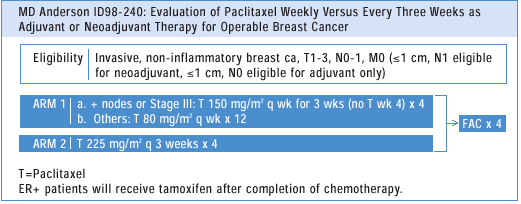
In the neoadjuvant
setting, we see about twice as many pathological complete responses
in both the breast and lymph nodes in patients who received a fractionated
weekly dose of paclitaxel compared to once every three weeks.In
terms of choosing a taxane, both Taxotere® (docetaxel) and Taxol
have substantial anti-tumor activity, but there are different safety
issues, and I have found that Taxol can be continued for a longer
time period.
—Aman
Buzdar, MD
SELECT
PUBLICATIONS
|
Alexandre
J et al. Factors predicting for efficacy and safety of
docetaxel in a compassionate-use cohort of 825 heavily pretreated
advanced breast cancer patients. J Clin Oncol 2000;18:562-73.
Abstract
Ando M et al. Efficacy of docetaxel 60 mg/m2
in patients with metastatic breast cancer according to the
status of anthracycline resistance. J Clin Oncol 2001;19:336-42.
Abstract
Bellon JR et al. Concurrent radiation therapy and paclitaxel
or docetaxel chemo-therapy in high-risk breast cancer.
Int J Radiat Oncol Biol Phys 2000;48:393-7. Abstract
Burstein HJ et al. Docetaxel administered on a weekly basis
for metastatic breast cancer. J Clin Oncol 2000;18:1212-9.
Abstract
Esteva FJ et al. Chemotherapy of metastatic breast cancer:
What to expect in 2001 and beyond. Oncologist 2001;6:133-46.
Abstract
Michaud
LB et al. Risks and benefits of taxanes in breast and ovarian
cancer. Drug Saf 2000;23:401-28. Abstract
Nabholtz JM et al. Docetaxel (Taxotere) in combination
with anthracyclines in the treatment of breast cancer. Semin
Oncol 2000;27:11-8. Abstract
Nabholtz
JM et al. Chemotherapy of breast cancer: Are the taxanes
going to change the natural history of breast cancer? Expert
Opin Pharmacother 2000;1:187-206. Abstract
Ravdin PM. Emerging role of docetaxel (Taxotere) in the
adjuvant therapy of breast cancer. Semin Oncol 1999;26:20-3.
Abstract
|
OTHER
RESOURCES
Taxanes in Cancer
Treatment: An NCI Publication for Patients Full
Text
|
|
