
 |
||||||||||||||

| Tracks 1-5 | ||||||||||||
|
Select Excerpts from the Interview
Track 2
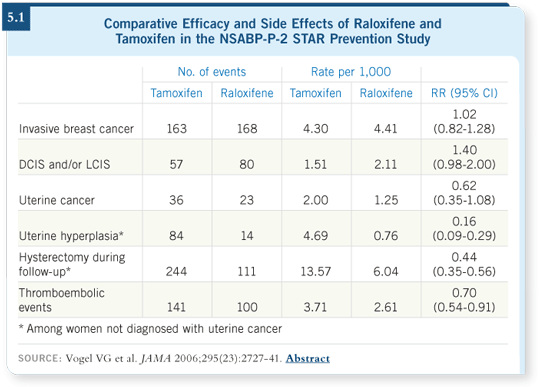
![]() DR WICKERHAM: The primary objective was to compare the effectiveness of
tamoxifen and raloxifene in the prevention of primary invasive breast cancer
for postmenopausal women at high risk. The results are clear that these drugs
are equally effective. Overall, the safety profile of raloxifene appears to be
better (5.1). The primary hope was that it would not increase the risk of
endometrial cancer.
DR WICKERHAM: The primary objective was to compare the effectiveness of
tamoxifen and raloxifene in the prevention of primary invasive breast cancer
for postmenopausal women at high risk. The results are clear that these drugs
are equally effective. Overall, the safety profile of raloxifene appears to be
better (5.1). The primary hope was that it would not increase the risk of
endometrial cancer.
Although the difference didn’t quite reach statistical significance, it’s clear that raloxifene has less of an impact on the endometrium. More than 50 percent of the women coming into the STAR trial had prior hysterectomies, and that is not by chance.
Not only did the women have a Gail model score given to them to qualify for the trial, we also gave them an estimate of their benefit and risk of entering the trial. It’s obvious that if you don’t have a uterus, you are not at risk for endometrial cancer. So in many ways we were selecting for the absence of a uterus, but that 50 percent reduction lowers the power to demonstrate no excess in endometrial cancer.
During the course of the trial, more than twice as many hysterectomies for benign conditions were performed among the tamoxifen-treated women, further reducing the ability to show a difference. Hyperplasia was 84 percent higher in the tamoxifen-treated group. The atypical hyperplasias were 12 to one comparing tamoxifen to raloxifene, and all these facts are consistent with the lack of an endometrial risk associated with raloxifene (5.1).
Fewer cataracts and fewer thromboembolic events, DVTs and pulmonary emboli were also observed with raloxifene. This was the first head-to-head comparison of tamoxifen and raloxifene, and it appears that raloxifene has a lowered risk of thromboembolic events compared to tamoxifen. So these findings combine to make raloxifene a more attractive drug in the prevention of this disease.
![]() DR LOVE: What about the incidence of DCIS?
DR LOVE: What about the incidence of DCIS?
![]() DR WICKERHAM: In the STAR trial, raloxifene wasn’t as effective as tamoxifen
in the reduction of LCIS and DCIS. The magnitude of that difference is
relatively small, and the clinical impact remains to be seen. It may have no
clinical impact, but it’s biologically intriguing. How could a drug be effective
in preventing invasive disease but be less effective on the precursors of that
invasive disease?
DR WICKERHAM: In the STAR trial, raloxifene wasn’t as effective as tamoxifen
in the reduction of LCIS and DCIS. The magnitude of that difference is
relatively small, and the clinical impact remains to be seen. It may have no
clinical impact, but it’s biologically intriguing. How could a drug be effective
in preventing invasive disease but be less effective on the precursors of that
invasive disease?
| Table of Contents | Top of Page |