
 |
||||||||||||||

| Tracks 1-7 | ||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR RAVDIN: A recent publication in the JCO described a study of the ability
of that test to predict sensitivity to chemotherapy (Paik 2006). The study was
conducted in collaboration with the NSABP.
DR RAVDIN: A recent publication in the JCO described a study of the ability
of that test to predict sensitivity to chemotherapy (Paik 2006). The study was
conducted in collaboration with the NSABP.
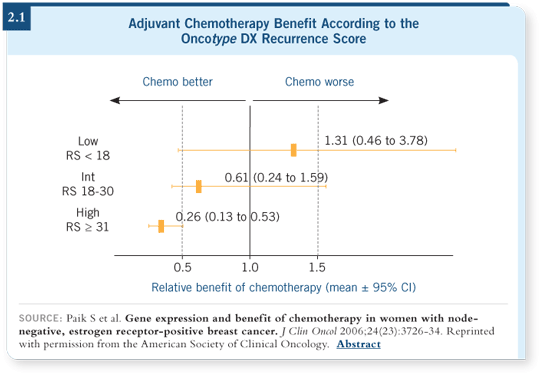
They found that patients with a low recurrence score appeared not to benefit from chemotherapy, but the patients who had high recurrence scores clearly were substantial winners in receiving adjuvant treatment.
In that trial, the absolute risk of distant recurrence in 10 years was reduced by roughly 30 percent among patients with high recurrence scores who received chemotherapy. For the patients with low recurrence scores, the risk of recurrence was similar between the groups, irrespective of whether they received chemotherapy. So that group didn’t appear to benefit (2.1).
![]() DR LOVE: What are the clinical situations in which you think the Oncotype
DX can be most useful?
DR LOVE: What are the clinical situations in which you think the Oncotype
DX can be most useful?
![]() DR RAVDIN: This test was designed to be used by and was developed with patients who have node-negative, estrogen receptor-positive disease.
DR RAVDIN: This test was designed to be used by and was developed with patients who have node-negative, estrogen receptor-positive disease.
Like any test, the Oncotype DX assay should be used when the result might affect the treatment decision. For most of us, irrespective of what a recurrence score showed, if a patient had a T3 tumor, we simply wouldn’t be satisfied with relying on the Oncotype test result.
Track 4
![]() DR RAVDIN: I believe we are at a transition point, and I expect it will become
more and more clear that aromatase inhibitors are the way to go. The reason
why we’re at a transition point is that, up until this time, the improvements
with aromatase inhibitors have been mainly limited to disease-free survival.
Individual trials and meta-analyses are now showing that this is converting
into an overall survival benefit (Mauri 2006).
DR RAVDIN: I believe we are at a transition point, and I expect it will become
more and more clear that aromatase inhibitors are the way to go. The reason
why we’re at a transition point is that, up until this time, the improvements
with aromatase inhibitors have been mainly limited to disease-free survival.
Individual trials and meta-analyses are now showing that this is converting
into an overall survival benefit (Mauri 2006).
These follow-up data strengthen the major guidelines from agencies in the United States, which now say that adjuvant therapy for a postmenopausal woman with ER-positive disease should include an aromatase inhibitor. The guidelines don’t specify that it is best to start with and to administer five years of aromatase inhibitors.
Many open questions have arisen. One trial will address whether you should start with an aromatase inhibitor and switch to tamoxifen. The other question that occurs to all of us is the follow-up question to the one that we faced 10 years ago with tamoxifen: If five years is good, is 10 years better? We don’t have any data to address that issue yet, but ongoing clinical trials are investigating what to do after five years of therapy with an aromatase inhibitor.
Track 7
![]() DR RAVDIN: Basically, aromatase inhibitor profiles look better than tamoxifen.
If you consistently look across studies, you see that the dropout rate is
always higher in the tamoxifen arm than it is in the aromatase inhibitor arm.
That tells you right away that the tolerability of the drug is at least as good as
tamoxifen. It’s not a dramatic difference, but it is always in favor of the aromatase
inhibitor.
DR RAVDIN: Basically, aromatase inhibitor profiles look better than tamoxifen.
If you consistently look across studies, you see that the dropout rate is
always higher in the tamoxifen arm than it is in the aromatase inhibitor arm.
That tells you right away that the tolerability of the drug is at least as good as
tamoxifen. It’s not a dramatic difference, but it is always in favor of the aromatase
inhibitor.
To me, that speaks deeply. We can all talk about aromatase inhibitor side effects like the arthralgias, and to be frank, the number of arthralgias you see depends on how hard you look. The real question is whether or not the patient had to stop the medication because she just couldn’t tolerate it.
![]() DR LOVE: Can you contrast the more serious side effects of the two drugs?
DR LOVE: Can you contrast the more serious side effects of the two drugs?
![]() DR RAVDIN: Tamoxifen increases the risk of thrombotic events and endometrial
cancer. Neither of those is an issue using an aromatase inhibitor. Tamoxifen
confers a benefit, which is that it seems to help retain bone mass. Aromatase
inhibitors tend to accelerate bone loss, and every single trial you can
review shows a trend — not a dramatic trend but about a one third increase in
the number of fractures that patients experience.
DR RAVDIN: Tamoxifen increases the risk of thrombotic events and endometrial
cancer. Neither of those is an issue using an aromatase inhibitor. Tamoxifen
confers a benefit, which is that it seems to help retain bone mass. Aromatase
inhibitors tend to accelerate bone loss, and every single trial you can
review shows a trend — not a dramatic trend but about a one third increase in
the number of fractures that patients experience.
In the trials, it’s well documented that people receiving aromatase inhibitors are losing bone mass faster, but growing evidence suggests that the use of bisphosphonates can block that effect.
| Table of Contents | Top of Page |