
 |
||||||||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 6
![]() DR FOX: Dr Oktay from Cornell has been a driving force behind a strategy
to stimulate the ovary to produce eggs for fertilization and cryopreservation
(Sonmezer, Oktay 2006).
DR FOX: Dr Oktay from Cornell has been a driving force behind a strategy
to stimulate the ovary to produce eggs for fertilization and cryopreservation
(Sonmezer, Oktay 2006).
Dr Oktay’s hypothesis is that if a patient is diagnosed with a breast cancer and is desirous of subsequently having children, you can stimulate her ovaries to produce eggs for fertilization before chemotherapy is initiated. Tamoxifen or letrozole is incorporated to stimulate the ovaries. The eggs can be retrieved in large numbers and can be fertilized, cryopreserved, and then implanted at a later time.
The concerns are whether this stimulatory strategy prior to chemotherapy will put the patient in harm’s way and whether the delay in chemotherapy, even by a few weeks, increases the patient’s risk of recurrence.
Even though it’s very early follow-up — approximately 18 months — and the number of patients is relatively small, he demonstrated as convincingly as one can in a small cohort that no precipitous increase in relapse rates occurred during that time interval.
We don’t yet know the fertility success rate. With only 18 months of data, those numbers are just beginning to develop.
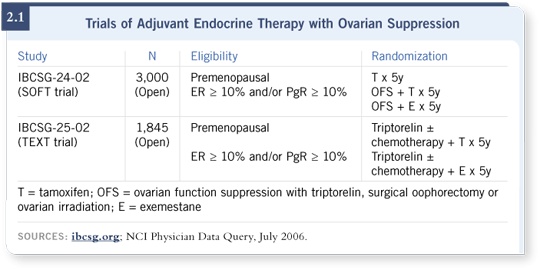
Track 11
![]() DR FOX: The biggest challenge in developing new therapeutic strategies
for premenopausal women with hormone receptor-positive breast cancers is
related to the issue of ovarian suppression. We are participating in one of the
largest clinical trials addressing this issue — the SOFT trial (2.1).
DR FOX: The biggest challenge in developing new therapeutic strategies
for premenopausal women with hormone receptor-positive breast cancers is
related to the issue of ovarian suppression. We are participating in one of the
largest clinical trials addressing this issue — the SOFT trial (2.1).
This trial randomly assigns premenopausal women with receptor-positive cancers to receive tamoxifen alone, ovarian suppression for five years with tamoxifen or ovarian suppression for five years with exemestane.
I don’t know of a more important question in clinical research on breast cancer in younger patients at the moment. The irony is that it’s been enormously difficult to get patients to participate in this trial, and accrual to the SOFT trial has been sluggish at best.
In the summary of recommendations of the 2005 St Gallen meeting, a little section appears at the end on the treatment of premenopausal women, worded in a very interesting way. It basically says that despite the absence of available data, the use of ovarian function suppression in premenopausal women is acceptable.
My feeling is that it certainly is acceptable. But acceptability notwithstanding, this is something that we at Penn have not offered to patients on a consistent basis for the simple reason that I am uncomfortable in the absence of supporting data.
I will say that, outside of a clinical trial like the SOFT or TEXT trial, we have not consistently offered ovarian suppression to patients in addition to their tamoxifen therapy. I still believe tamoxifen is the standard of care.
Track 13
![]() DR FOX: At the moment, you have to look at patients based on where they
happen to be in their course of treatment.
DR FOX: At the moment, you have to look at patients based on where they
happen to be in their course of treatment.
The ATAC trial addresses treatment of the newly diagnosed postmenopausal patient with estrogen receptor-positive breast cancer, and I believe this trial gives irrefutable evidence that anastrozole is superior to tamoxifen with respect to reducing the risk of recurrence. So for the newly diagnosed patient, the available data suggest that five years of an aromatase inhibitor is the best therapy (Howell 2005).
The preliminary information with letrozole in the BIG 1-98 study gives the same message (Thürlimann 2005). The follow-up is shorter — a little over two years versus six in ATAC — but the apparent reduction in the risk of recurrence is on the order of that seen with anastrozole. I think that most of us believe them to be likely equivalent. The preferential prescription of anastrozole at the moment is based on more maturity of data.
The second situation is that of the patient in the middle of a course of therapy. This was evaluated in the international exemestane group trial and the trials of anastrozole, which were similarly constructed (Coombes 2005; Boccardo 2005; Jakesz 2005). These trials were designed to capture patients at the midpoint of a course of therapy and measure outcomes from the point of changing treatment, randomly assigning patients to continue tamoxifen or take an aromatase inhibitor for the balance of the five-year period.
For the patient in the middle of a course of tamoxifen therapy or the premenopausal woman who’s become amenorrheic from chemotherapy and has been on tamoxifen and amenorrheic for two years, it is appropriate to change her to an aromatase inhibitor.
A third situation is that of the patient who has completed five years of tamoxifen. MA17 demonstrates that letrozole produces a small but measurable reduction in the risk of recurrence and an indication of a survival benefit among women with node-positive disease (Goss 2005, 2006).
If you evaluate patients with respect to where they fall in time and follow the data that exist, these are the three scenarios and the three approaches I would take.
| Table of Contents | Top of Page |
Editor:
Neil Love, MD
Interviews
Monica Morrow, MD
- Select publications
Kevin R Fox, MD
- Select publications
Thomas B Julian, MD
- Select publications
Robert W Carlson, MD
- Select publications
Richard M Elledge, MD
- Select publications