
 |
||||||||||||||

| Tracks 1-6 | ||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-4
![]() DR ELLEDGE: I believe it is a very useful tool. I would strongly endorse
community oncologists using it in situations in which they are trying to
decide whether to use chemotherapy. The strengths of the Oncotype DX assay
are its standardization and reproducibility.
DR ELLEDGE: I believe it is a very useful tool. I would strongly endorse
community oncologists using it in situations in which they are trying to
decide whether to use chemotherapy. The strengths of the Oncotype DX assay
are its standardization and reproducibility.
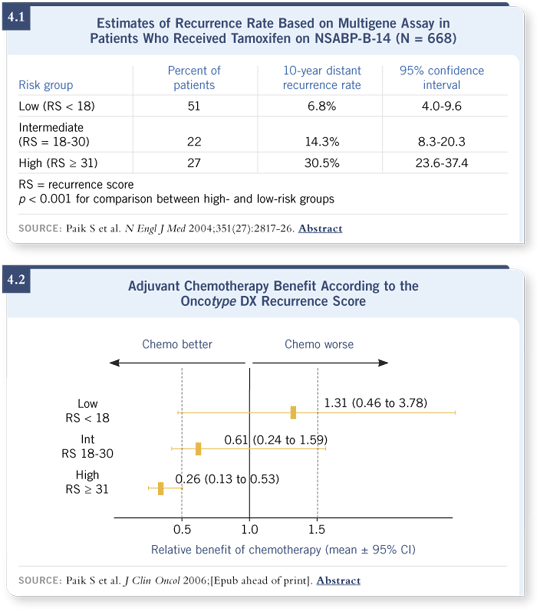
The assay uses a collection of genes that were combed from the world’s literature over the last 30 years and found to be associated with outcome in breast cancer. They were combined in an assay and tested retrospectively in databases to see how they predicted for the natural history of the disease after local therapies (Paik 2004; [4.1]) and for response to adjuvant chemotherapy (Paik 2006; [4.2]).
The Oncotype DX assay offers prognostic information about the risk of recurrence. It presents a visual representation of where your patient lies along a spectrum. It will assign a score, which some people “trichotomize” into a low, intermediate or high score. Patients who are on the lower end of the spectrum will have a lower absolute risk of recurrence and much lower benefit from chemotherapy (4.1, 4.2).
Tracks 5-6
![]() DR ELLEDGE: Quality control is crucial in measuring these markers. We do
not have good quality control throughout the United States. That has been
clearly shown multiple times objectively, for both ER and HER2.
DR ELLEDGE: Quality control is crucial in measuring these markers. We do
not have good quality control throughout the United States. That has been
clearly shown multiple times objectively, for both ER and HER2.
For instance, at the beginning of NSABP-B-31, the NCI and Soon Paik showed that the risk of an error in HER2 assessment was 24 percent, especially from smaller labs (Paik 2002). For ER, studies in the United States and Europe have shown that ER testing is inaccurate in the 20 percent range, especially for ER negativity. This has clouded the results of our studies and our thinking. So standardization is extremely important.
It’s very difficult for community oncologists to become involved because this is a pathology issue. In terms of practical advice, I tell them that I would insist that their patients’ breast tumors be sent to large reference labs. I believe that if you measure ER and HER2 accurately and ER is clearly positive and HER2 is clearly negative, the benefits from chemotherapy are modest at best and may be nonexistent.
![]() DR LOVE: The flip side is the patient who is not receiving therapy because of
inaccuracies in how her tumor was studied — women who are said to have
ER-negative tumors when in fact their tumors are ER-positive and they don’t
receive hormone therapy and, likewise, patients who are said to have HER2-negative disease when they have HER2-positive disease and who don’t receive
trastuzumab. It surprises me that people aren’t more upset about this.
DR LOVE: The flip side is the patient who is not receiving therapy because of
inaccuracies in how her tumor was studied — women who are said to have
ER-negative tumors when in fact their tumors are ER-positive and they don’t
receive hormone therapy and, likewise, patients who are said to have HER2-negative disease when they have HER2-positive disease and who don’t receive
trastuzumab. It surprises me that people aren’t more upset about this.
![]() DR ELLEDGE: It has surprised me too. In my editorial published in the JCO,
I calculated that up to 1,000 women per year die because of inaccurate ER
assays (Elledge 2006; [4.3]).
DR ELLEDGE: It has surprised me too. In my editorial published in the JCO,
I calculated that up to 1,000 women per year die because of inaccurate ER
assays (Elledge 2006; [4.3]).

| Table of Contents | Top of Page |
Editor:
Neil Love, MD
Interviews
Monica Morrow, MD
- Select publications
Kevin R Fox, MD
- Select publications
Thomas B Julian, MD
- Select publications
Robert W Carlson, MD
- Select publications
Richard M Elledge, MD
- Select publications