
| Tracks 1-10 |
| Track 1 |
Introduction |
| Track 2 |
Effect of preoperative aromatase
inhibitors on assays of Ki-67 |
| Track 3 |
Clinical trials of the aromatase
inhibitors for DCIS and prevention |
| Track 4 |
Hormonal therapy options for
premenopausal patients with
ER-positive disease |
| Track 5 |
Impact of the aromatase inhibitors
on bone mineral density and
fracture risk |
|
| Track 9 |
Optimal duration of adjuvant
endocrine therapy |
| Track 10 |
Use of genetic profiling in the
neoadjuvant setting to predict
response to aromatase inhibitors |
| Track 11 |
Neoadjuvant trials for the
development of novel therapeutic
agents |
| Track 12 |
Current status of sentinel lymph
node biopsy |
| Track 13 |
Partial breast irradiation |
|
|
Select Excerpts from the Interview
 Track 2
Track 2
 DR LOVE: Would you discuss your trial of neoadjuvant aromatase
inhibitors? DR LOVE: Would you discuss your trial of neoadjuvant aromatase
inhibitors? |
 DR DIXON: We conducted a study in women with invasive breast cancer: 206
patients with 209 tumors. The patients were randomly assigned to receive 14
days of either letrozole or anastrozole, preoperatively. In terms of switching off
cell proliferation, we couldn't find any significant difference between anastrozole
and letrozole (Murray 2004; Faratian 2005).
DR DIXON: We conducted a study in women with invasive breast cancer: 206
patients with 209 tumors. The patients were randomly assigned to receive 14
days of either letrozole or anastrozole, preoperatively. In terms of switching off
cell proliferation, we couldn't find any significant difference between anastrozole
and letrozole (Murray 2004; Faratian 2005).
 DR LOVE: Can you talk about the data that have been presented on the effect
of preoperative endocrine therapy on Ki-67?
DR LOVE: Can you talk about the data that have been presented on the effect
of preoperative endocrine therapy on Ki-67?
 DR DIXON: Mitch Dowsett presented data from the IMPACT trial at the
2005 San Antonio Breast Cancer Symposium showing that the 14-day Ki-67
predicts relapse-free survival (Dowsett 2005).
DR DIXON: Mitch Dowsett presented data from the IMPACT trial at the
2005 San Antonio Breast Cancer Symposium showing that the 14-day Ki-67
predicts relapse-free survival (Dowsett 2005).
What’s interesting about the research is that it validated the idea that if your
proliferation goes down or is low after two weeks on a drug, then you will
have a better long-term outcome. If you don’t have any decrease in proliferation, that indicates your cancer is resistant to endocrine therapy (Dowsett
2005).
 DR LOVE: Does the same correlation hold true with chemotherapy?
DR LOVE: Does the same correlation hold true with chemotherapy?
 DR DIXON: That has been investigated, but the problem with chemotherapy,
compared to endocrine therapy, is sampling time. Chemotherapy is administered
in pulses, and the proliferation rate drops within a few days.
DR DIXON: That has been investigated, but the problem with chemotherapy,
compared to endocrine therapy, is sampling time. Chemotherapy is administered
in pulses, and the proliferation rate drops within a few days.
So the question is, when do you sample to find how it is impacting the tumor?
In terms of sampling for Ki-67, the favorable aspect of endocrine therapy is
that it is administered constantly.
 DR LOVE: In your study of women with invasive cancers, what fraction of
ER-positive tumors drop in proliferation at two weeks?
DR LOVE: In your study of women with invasive cancers, what fraction of
ER-positive tumors drop in proliferation at two weeks?
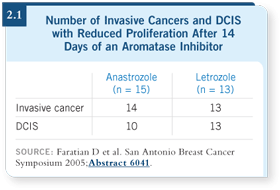
 DR DIXON: About 90 percent of the patients showed a drop at two weeks
(2.1). In that study, we also had our pathologists look through the core biopsies
and the final histologies to determine how many of them had DCIS. Then
they looked at the effects of the aromatase inhibitors on the DCIS.
DR DIXON: About 90 percent of the patients showed a drop at two weeks
(2.1). In that study, we also had our pathologists look through the core biopsies
and the final histologies to determine how many of them had DCIS. Then
they looked at the effects of the aromatase inhibitors on the DCIS.
Surprisingly, we found that DCIS was proliferating as much as the invasive
cancer (Faratian 2005). The second observation was that the DCIS was proliferating
at roughly the same rate as the invasive cancer in an individual patient.
In other words, if your cancer was highly proliferative, your DCIS was highly
proliferative (Faratian 2005).
 DR LOVE: Were the aromatase
inhibitors having an
effect on the DCIS?
DR LOVE: Were the aromatase
inhibitors having an
effect on the DCIS?
 DR DIXON: We could not
tell whether the aromatase
inhibitors were eliminating
the DCIS, but we could see
they were remarkably effective
at switching off proliferation
in the DCIS (2.1).
DR DIXON: We could not
tell whether the aromatase
inhibitors were eliminating
the DCIS, but we could see
they were remarkably effective
at switching off proliferation
in the DCIS (2.1).
We had approximately an 80
percent switch-off of proliferation.
If you measure the
level at the start, at 100 percent, it was down to 20 percent within a couple of
weeks (Faratian 2005).
We saw other biological effects, too. For example, the progesterone receptor
was switched off (Faratian 2005). These were potent biological effects on
DCIS.
To some extent, this starts to provide us with an insight as to why the aromatase
inhibitors are probably more effective at stopping other cancers from
developing, because they work on these earlier lesions.
Although some studies are now evaluating the aromatase inhibitors for patients
with DCIS, if I were a patient with DCIS, then I’d be thinking that an aromatase
inhibitor might be a good idea.
 Track 4
Track 4
 DR LOVE: What do you think about the strategy of ovarian suppression
and an aromatase inhibitor for a premenopausal patient with node-positive,
ER-positive, HER2-positive disease? DR LOVE: What do you think about the strategy of ovarian suppression
and an aromatase inhibitor for a premenopausal patient with node-positive,
ER-positive, HER2-positive disease? |
 DR DIXON: It sounds sensible because we know the aromatase inhibitors are
effective in patients with HER2-positive disease. In our preoperative study,
we found the aromatase inhibitors were as effective at reducing proliferation in
patients with HER2-positive disease as in those with HER2-negative disease.
The degree of reduction was identical in patients with HER2-positive and
HER2-negative disease (Murray 2004).
DR DIXON: It sounds sensible because we know the aromatase inhibitors are
effective in patients with HER2-positive disease. In our preoperative study,
we found the aromatase inhibitors were as effective at reducing proliferation in
patients with HER2-positive disease as in those with HER2-negative disease.
The degree of reduction was identical in patients with HER2-positive and
HER2-negative disease (Murray 2004).
It’s as though HER2 isn’t important in relation to the likelihood of responding
to an aromatase inhibitor.
 Track 5
Track 5
 DR LOVE: There was an increased rate of fractures associated with
anastrozole in the ATAC trial, but they didn’t monitor bone density or use
bisphosphonates. What is your approach to monitoring bone density in
patients on aromatase inhibitors? DR LOVE: There was an increased rate of fractures associated with
anastrozole in the ATAC trial, but they didn’t monitor bone density or use
bisphosphonates. What is your approach to monitoring bone density in
patients on aromatase inhibitors? |
 DR DIXON: If you have a drug that is more effective against breast cancer and
it causes some minor problems, then I’d rather circumvent the problems and
utilize the more effective drug.
DR DIXON: If you have a drug that is more effective against breast cancer and
it causes some minor problems, then I’d rather circumvent the problems and
utilize the more effective drug.
One of the clinical applications to arise from the IBIS trial is an easy way to
manage bone density in patients on aromatase inhibitors. Rob Coleman, who
is a bone expert in the United Kingdom, has developed an algorithm that’s
very straightforward. If you’re starting a woman on five years of an adjuvant
aromatase inhibitor, you need to check the bone density beforehand and at
regular intervals.
If you’re switching women from adjuvant tamoxifen after two to three years to
an aromatase inhibitor, you don’t really need to bother with the bone density
between the ages of 50 and 64. After 64 years of age, you should obtain a
DEXA scan at the time of the switch.
 Track 6
Track 6
 DR LOVE: Do you think it’s justifiable to use more than a couple of years
of adjuvant tamoxifen in a postmenopausal patient with an invasive ER-positive
tumor? DR LOVE: Do you think it’s justifiable to use more than a couple of years
of adjuvant tamoxifen in a postmenopausal patient with an invasive ER-positive
tumor? |
 DR DIXON: For the majority of women who are on tamoxifen now, it’s best to
switch them to an aromatase inhibitor. For those who are reaching the end of
five years on tamoxifen, I would continue them on it and then use extended
adjuvant therapy.
DR DIXON: For the majority of women who are on tamoxifen now, it’s best to
switch them to an aromatase inhibitor. For those who are reaching the end of
five years on tamoxifen, I would continue them on it and then use extended
adjuvant therapy.
The issue is, of course, how long do you switch them for?
One of the things that the MA17 trial has shown us is that five years of treatment
is not enough (Goss 2005). So will we use only five years of an aromatase
inhibitor? Should we continue the aromatase inhibitor beyond that? Should a woman who was treated with two to three years of tamoxifen receive
five, rather than two to three, years of an aromatase inhibitor?
I believe we will find that the overall length of treatment will not be five
years but that we will need to use a longer duration.
 DR LOVE: An NSABP study will evaluate five years of an aromatase inhibitor
beyond the initial five years or in patients who have switched to an aromatase
inhibitor at two years who are now five years past their surgery.
DR LOVE: An NSABP study will evaluate five years of an aromatase inhibitor
beyond the initial five years or in patients who have switched to an aromatase
inhibitor at two years who are now five years past their surgery.
 DR DIXON: I believe the studies evaluating more prolonged endocrine
therapies are likely to show a benefit.
DR DIXON: I believe the studies evaluating more prolonged endocrine
therapies are likely to show a benefit.
One of the reasons I’m sure they will is because the aromatase inhibitors are
very good preventive agents.
Among women who have undergone breast-conserving surgery, almost all
the recurrences after five years are second primaries, not recurrences. That’s
frustrating for me as a surgeon.
The patient is doing well for five, six, seven years, and suddenly she springs
up another cancer. She needs to be treated again, and it’s devastating for the
woman.
So if we can continue her on a drug that suppresses the rate of new cancers, I
believe that will be tremendous.
Select publications

