
| Tracks 1-15 |
| Track 1 |
Introduction |
| Track 2 |
Margin status: Impact on re-excision and local control |
| Track 3 |
Partial breast irradiation techniques being evaluated in clinical trials |
| Track 4 |
Postlumpectomy radiation therapy for women over the age of 70 |
| Track 5 |
Postlumpectomy radiation therapy and hormonal therapy for women with DCIS |
| Track 6 |
Perspective on the role of aromatase inhibitors in the prevention and
adjuvant settings |
| Track 7 |
Side effects associated with aromatase inhibitors |
| Track 8 |
Case 1: A patient with a low recurrence score on the Oncotype DX™ assay |
|
| Track 9 |
Case 2: A patient with a high recurrence score on the Oncotype DX assay |
| Track 10 |
Cost effectiveness of the Oncotype DX assay |
| Track 11 |
Sentinel lymph node biopsy |
| Track 12 |
Axillary dissection after identification of a positive sentinel node |
| Track 13 |
Considerations in the selection of patients for nipple-sparing mastectomy |
| Track 14 |
False-negative rates in sentinel node biopsies |
| Track 15 |
Surgical volume and outcomes for patients with breast cancer |
|
|
Select Excerpts from the Interview
 Track 2
Track 2
 DR LOVE: What do you consider an acceptable surgical margin in breast cancer? DR LOVE: What do you consider an acceptable surgical margin in breast cancer? |
 DR BORGEN: Nationally, no consensus exists on what constitutes an acceptable margin with either ductal carcinoma in situ (DCIS) or invasive carcinoma. I believe it does a disservice to insist on one-millimeter margins or four-millimeter margins.
DR BORGEN: Nationally, no consensus exists on what constitutes an acceptable margin with either ductal carcinoma in situ (DCIS) or invasive carcinoma. I believe it does a disservice to insist on one-millimeter margins or four-millimeter margins.
For example, if you have a single duct with DCIS a millimeter or two from a margin, in my opinion that margin is okay. However, if you have a field of ducts, all of which are one millimeter from a margin, the pathology report will still read, “DCIS, one millimeter from a margin,” but the tumor burden at that margin makes it very likely that disease will be left behind.
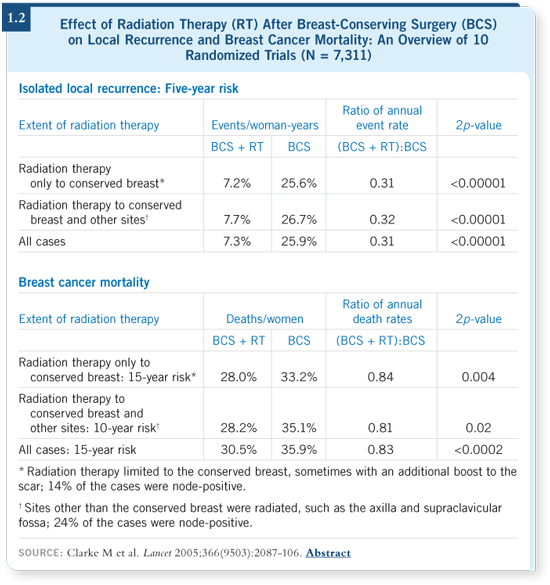
 DR LOVE: How has the Oxford Overview on the effects of radiation therapy and surgery affected your view on the importance of margins and local control (Clarke 2005)?
DR LOVE: How has the Oxford Overview on the effects of radiation therapy and surgery affected your view on the importance of margins and local control (Clarke 2005)?
 DR BORGEN: Margins always come into play, whether we’re dealing with invasive or in situ breast cancer. One of the outcomes of this overview should be that surgeons pay more attention to margins and local control so that we don’t have patients slipping through the cracks and not receiving radiation therapy.
DR BORGEN: Margins always come into play, whether we’re dealing with invasive or in situ breast cancer. One of the outcomes of this overview should be that surgeons pay more attention to margins and local control so that we don’t have patients slipping through the cracks and not receiving radiation therapy.
Nationally, the re-excision rates approach 50 percent, and at Memorial, almost half our patients go back for more surgery. We’re currently evaluating whether we can design a smarter operative field with preoperative MRIs.
However, this is a problem because MRI is an expensive technology and not everyone has access to it. Also, the MRI can display phantoms and has a relatively high false-positive rate associated with it.
The Oxford Overview raises the bar on the value of local control, which causes some concern in the national trials of partial breast radiation therapy. I’m not a naysayer. In fact, we are participating in the NSABP-B-39 trial.
However, as a cautionary note, whole breast radiation now has an established track record in a meta-analysis, with proven deleterious effects among patients who did not receive whole breast radiation (1.1, 1.2).
 DR LOVE: Can you elaborate on how you assess margins and make decisions about whether to re-excise?
DR LOVE: Can you elaborate on how you assess margins and make decisions about whether to re-excise?
 DR BORGEN: We have recently changed our approach to margins. In the past, we performed a lumpectomy, either by palpation or by image guidance with a wire, and oriented that specimen in space with silk sutures. The pathologists then applied six different colors of ink to the mass that we removed.
DR BORGEN: We have recently changed our approach to margins. In the past, we performed a lumpectomy, either by palpation or by image guidance with a wire, and oriented that specimen in space with silk sutures. The pathologists then applied six different colors of ink to the mass that we removed.
However, we have determined that this technique was not accurate. The definition of what was anterior or superior was left up to a pathologist who was not present during the surgical procedure and could not be certain.

Today when we do a lumpectomy, we shave the margins individually, intraoperatively.
We place a silk suture on the new margin, on the shave, so we know that the orientation of the specimen being sent to the pathologist is correctly identified.
This approach has been very successful. We’ve dropped our re-excision and positive-margin rates, and when we have to go back for a positive margin, we have been more successful in finding residual cancer.
 Track 5
Track 5
 DR LOVE: How are you treating patients with DCIS in terms of
endocrine therapy? DR LOVE: How are you treating patients with DCIS in terms of
endocrine therapy? |
 DR BORGEN: We have viewed tamoxifen as a highly appropriate option for
treating ER-positive DCIS since the NSABP-B-24 trial (Fisher 1999).
DR BORGEN: We have viewed tamoxifen as a highly appropriate option for
treating ER-positive DCIS since the NSABP-B-24 trial (Fisher 1999).
However, when we sit down and look at risks, benefits and quality-of-life
issues, it’s common for our New York patients to demur, so we probably have
one of the lowest percentages of patients with DCIS on tamoxifen in
the country.
The same can be seen in the prevention setting, in which we’ve not been
successful in getting patients to take tamoxifen.
 DR LOVE: What are the concerns about tamoxifen in these settings?
DR LOVE: What are the concerns about tamoxifen in these settings?
 DR BORGEN: The two most obvious concerns are endometrial cancer and
gynecological events.
DR BORGEN: The two most obvious concerns are endometrial cancer and
gynecological events.
Even when we provide the raw numbers on how infrequent those events are,
I believe that because we are talking about minimal, if any, impact on long-term
survivorship and moderate impact on local control, it simply is not an
attractive option.
 DR LOVE: For a postmenopausal patient with DCIS who is interested in
endocrine therapy but finds tamoxifen intolerable because of side effects, do
you offer an aromatase inhibitor?
DR LOVE: For a postmenopausal patient with DCIS who is interested in
endocrine therapy but finds tamoxifen intolerable because of side effects, do
you offer an aromatase inhibitor?
 DR BORGEN: We’d like to have more information about DCIS and aromatase
inhibitors, but since the initial publication of the ATAC data (Baum 2002),
aromatase inhibitors have certainly become our endocrine therapy of choice
for postmenopausal patients with ER-positive, invasive cancers.
DR BORGEN: We’d like to have more information about DCIS and aromatase
inhibitors, but since the initial publication of the ATAC data (Baum 2002),
aromatase inhibitors have certainly become our endocrine therapy of choice
for postmenopausal patients with ER-positive, invasive cancers.
That literally happened overnight, like gangbusters, and so a “bleedover” to
postmenopausal patients with DCIS is completely natural.
 Track 7
Track 7
 DR LOVE: What side effects have you observed in patients who are
receiving aromatase inhibitors? DR LOVE: What side effects have you observed in patients who are
receiving aromatase inhibitors? |
 DR BORGEN: Aches and pains — particularly of the knees and hips — are the
most common complaints. However, these are far less than the complaints we
heard from patients on tamoxifen.
DR BORGEN: Aches and pains — particularly of the knees and hips — are the
most common complaints. However, these are far less than the complaints we
heard from patients on tamoxifen.
 DR LOVE: In clinical practice, what is your protocol for monitoring bone
density and the use of bisphosphonates in patients on aromatase inhibitors?
DR LOVE: In clinical practice, what is your protocol for monitoring bone
density and the use of bisphosphonates in patients on aromatase inhibitors?
 DR BORGEN: If we are concerned about a bone density report, we will
refer the patient to an endocrinologist for further workup prior to beginning
aromatase inhibitor therapy.
DR BORGEN: If we are concerned about a bone density report, we will
refer the patient to an endocrinologist for further workup prior to beginning
aromatase inhibitor therapy.
In New York, patients are very proactive and they come into the office aware
of their bone density and, if there’s a problem, they are generally already on a
bisphosphonate or similar agent.
 DR LOVE: Have you utilized neoadjuvant aromatase inhibitors to downsize
tumors in order to convert a mastectomy to a lumpectomy?
DR LOVE: Have you utilized neoadjuvant aromatase inhibitors to downsize
tumors in order to convert a mastectomy to a lumpectomy?
 DR BORGEN: Absolutely. Certainly in the older patient population we have
utilized that approach. However, it’s not so much to convert a mastectomy to a
lumpectomy as it is to downstage the disease.
DR BORGEN: Absolutely. Certainly in the older patient population we have
utilized that approach. However, it’s not so much to convert a mastectomy to a
lumpectomy as it is to downstage the disease.
 Track 7
Track 7
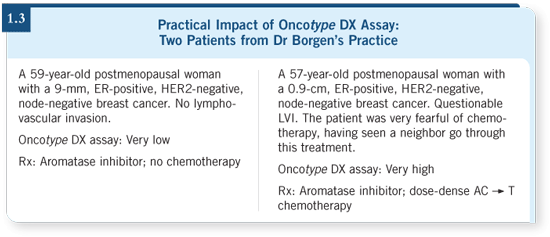
 DR LOVE: What is your opinion of the Oncotype DX assay, and how do
you utilize it clinically? DR LOVE: What is your opinion of the Oncotype DX assay, and how do
you utilize it clinically? |
 DR BORGEN: We’re very excited about the possibility of a truly genomic
approach to breast cancer. We use the Oncotype DX assay in borderline cases
in which a low recurrence score would preclude cytotoxic chemotherapy (Paik
2004; Mamounas 2005).
DR BORGEN: We’re very excited about the possibility of a truly genomic
approach to breast cancer. We use the Oncotype DX assay in borderline cases
in which a low recurrence score would preclude cytotoxic chemotherapy (Paik
2004; Mamounas 2005).
For the patient who has a larger tumor, a higher-grade tumor or other
mitigating factors, we’re not using the Oncotype DX as a sole factor in
precluding chemotherapy, but it’s been enormously helpful in the
borderline cases.
Select publications

