| You
are here: Home: Meet
The Professors Vol. 1 2003: Case
4
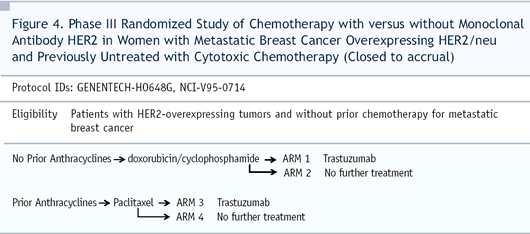
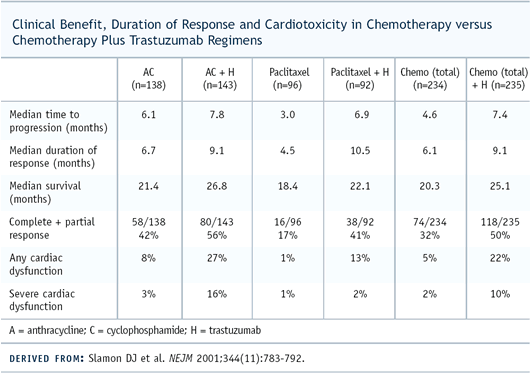
The paclitaxel/trastuzumab arm had improvements in response rate
and time to progression but, in that subset, there wasn’t
an overall survival advantage. You had to look at all 400-plus
patients in the trial to see an improvement in survival.
For this patient, I would recommend paclitaxel, carboplatin and
trastuzumab trial (Figure 5). We did a Phase III randomized trial,
building upon the results of the pivotal trial to try to improve
the outcome. In vitro data demonstrated that carboplatin is synergistic
with either paclitaxel or docetaxel. Our trial addressed the very
simple question: How does the addition of carboplatin affect outcomes?
We utilized the same schema used in the pivotal trial — paclitaxel
every three weeks at 175 mg/m2 and carboplatin with an AUC of six.
Our carboplatin dose was based on some of Edith’s work evaluating
paclitaxel/ carboplatin every three weeks. We were very pleased
that this laboratory work was carried through to the bedside.
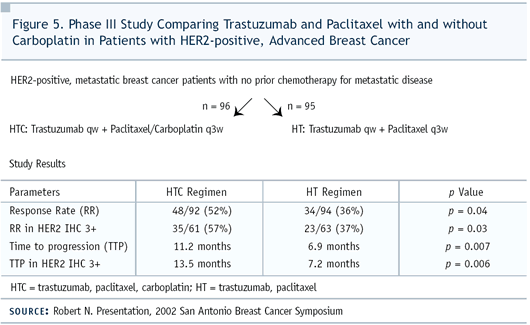
In our trial of patients with HER2-positive (IHC 3+) metastatic
disease, adding carboplatin to paclitaxel improved response rates
to about 57% and doubled the time to progression to 14 months.
We also saw an improvement of survival by nine months, although
it’s not statistically significant. I don’t expect
to see statistically significant improvement in survival — just
as it wasn’t seen in the paclitaxel arm of the original pivotal
trial — because we only had 191 patients. It’s also
interesting to note that it took less than two months to see a
response. This is a fast-acting regimen, considering that we don’t
look for measurable disease more than once every two months.
Another important factor is that trastuzumab, like endocrine
therapy, may be continued after chemotherapy. The pivotal trial
mandated six cycles of chemotherapy, and we designed our trial
in very much the same way. Three-quarters of the responding patients
stopped chemotherapy after six cycles. In this strategy, chemotherapy
is given in a synergistic combination with trastuzumab to induce
a remission — in the parlance of leukemia — and after
you have achieved the maximum response, then trastuzumab can be
continued as maintenance therapy.
Dr Love: What was the tolerability
of this combination regimen?
Dr Robert: Generally, this regimen
was well- tolerated. We were concerned about myelosuppression from
adding carboplatin to paclitaxel, and we did see more neutropenia
and thrombocytopenia but no neutropenic fever. There was not an
increased incidence of neuropathy. We saw slightly more fatigue,
but we did not control for treatment with erythropoietin, so we
cannot comment on whether or not this was related to anemia. It
is important to note that this was not a dose-dense regimen, and
we did not utilize prophylactic growth factors. If the counts were
not adequate, then we would dose-reduce or dose-delay, and that
partly explains the relatively favorable side-effect profile.
Dr Chittoor: When you give trastuzumab long-term, how do you
monitor the cardiac status? I’ve had three patients who didn’t
go into failure but had significant decreases in ejection fraction
(EF). I checked EFs every six months. I realize that it is touted
to be a reversible left ventricular dysfunction. Nonetheless, let
us presume you do stop it and the EFs improve. Can you restart
trastuzumab again?
Dr Perez: That’s a great
question. When trastuzumab first became available, the package
insert indicated that you should monitor left ventricular ejection
fraction. We were routinely doing those evaluations, but we have
completely abandoned that in our practice because we haven’t
seen any correlation between asymptomatic decreases of ejection
fraction and the development of congestive heart failure. Now the
patients are managed on the basis of their physical symptoms.
In terms of clinical trials, we are doing very intense monitoring
not only of left ventricular ejection fraction, but we are also
looking at potential serum or plasma markers that may be predictive.
Those studies are still in the investigational stages.
One of the challenges we have is that there is really not a database
telling us that there’s a good correlation even between MUGAs
and echos when we look at ejection fractions. There’s a vacuum
in knowledge, and that’s one of the reasons we’ve abandoned
routine evaluation of left ventricular ejection fraction.
It appears that in the few patients who have had endomyocardial
biopsies after the development of trastuzumab-induced congestive
heart failure, there is no documented anatomical vacuolization.
This is very different than what is seen in anthracycline-induced
cardiomyopathy, in which you see the vacuoles and can look at the
damage. With trastuzumab, there’s no damage that is readily
seen under the microscope. One of the theories is that this drug
may be associated with stunning the myocardium, and many patients
improve after decreases in ejection fraction.
One thing that we’re learning, just recently, is that this
can also happen with chemotherapy. We have become very comfortable
using anthracyclines, and we usually do not check ejection fractions.
At the ASCO meeting this year, there was a wealth of information
regarding this issue. We give AC x 4, the ejection fraction drops;
we follow ejection fraction, and a few months later that number
improves.
I think we have been sensitized to the issue of trastuzumab.
We’ve been seeing a lot of ejection fractions, and we don’t
really know how much that translates into the clinical symptomatology
of our patients.
Dr Robert: Increasingly, I’ve
heard that people are becoming more comfortable dropping the ventricular
ejection fraction evaluation. The argument has been, if trouble
arises, it will be early, within the first few months. That’s
a good reason to initiate that test in the first few months. Afterwards,
it doesn’t seem to be a problem — unlike with the use
of doxorubicin. What I haven’t heard is evidence that the
hypothesis is true — whether two or three years later, cardiac
functioning is okay. Now, there aren’t that many patients
who stay on trastuzumab or any regimen for years, but there are
a few. If I’m giving a patient a drug that may affect their
heart in a deleterious way, I should share those concerns with
the patient. For patients who are responding to trastuzumab but
develop congestive heart failure, you can continue trastuzumab
and treat their cardiac condition with diuretics and ACE inhibitors
and maintain a positive quality of life and good cardiac function.
Dr Love: Any comments from the
group about the new data that Dr Robert was discussing with regard
to carboplatin, docetaxel and trastuzumab?
Dr Firstenberg: We’ve
been a big proponent of using platinums — both cisplatin
and carboplatin — in patients with HER2-positive tumors,
and then we use trastuzumab and vinorelbine.
Dr Love: Edith, what went into
the evolution of using carboplatin versus cisplatin in some of
these studies?
Dr Perez: We selected carboplatin
based on the tolerability compared to cisplatin. A lower dosage
of cisplatin needs to be studied, but we don’t have as much
data on that drug, compared to carboplatin.
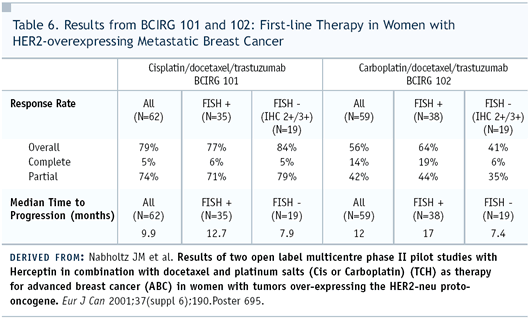
Dr Robert: The BCIRG has Phase
II experience, in which cisplatin and carboplatin were used in
combination with trastuzumab, and the response rates are comparable.
The patient populations are not the same. (Table 6) Patients who
received carboplatin had prior therapy, but the time to progression
was longer in the carboplatin group. It’s a little bit like
comparing “apples and oranges,” but I think all of
us are convinced that carboplatin is better-tolerated than cisplatin.
Dr Robert: Another question
for which we do not have an answer is how long should we continue
trastuzumab after first-line therapy? For example, a patient receives
carboplatin and paclitaxel, progresses, then you give them vinorelbine
and the tumor progresses. Does it make sense to continue evaluating
drugs that are either synergistic or additive? Is it time to stop?
We don’t have any evidence to direct us. One trial attempted
to take patients who failed trastuzumab/taxane therapy and randomize
them to vinorelbine versus vinorelbine and trastuzumab. That trial
had poor accrual and it’s closed. It may resurface as an
Intergroup trial.
Another issue is the schedule of trastuzumab. We have pharmacokinetic
data from Brian Leyland-Jones that supports giving trastuzumab
every three weeks instead of weekly, so our patients do not have
to come into the office every week. This was a proof of principle
trial, so we didn’t become involved with what was the best
schedule for administering paclitaxel or carboplatin. We just wanted
to demonstrate that adding carboplatin made a difference. Clearly,
there are scheduling questions, and Dr Perez can tell us about
her experience using weekly paclitaxel/carboplatin and trastuzumab,
as opposed to the every-three-week schedule.
Dr Perez: At the NCCTG we have
been interested in the taxane/carboplatin and the paclitaxel/carboplatin/trastuzumab
combinations for a few years. We are completing a randomized Phase
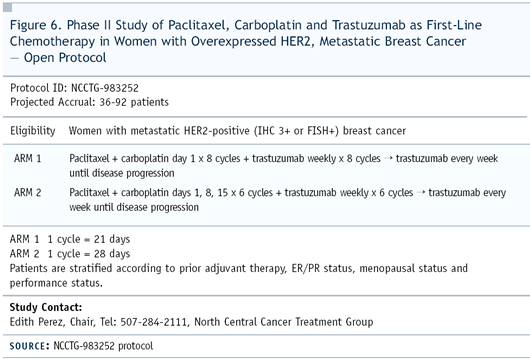
II trial — NCCTG-983252 — in which the basic question
was the scheduling of the three drugs (Figure 6).
Dr Robert and colleagues undertook a study that would answer
the question of how much carboplatin should be added to paclitaxel/trastuzumab,
so we elected to look at paclitaxel/carboplatin/trastuzumab every-three-weeks
versus given on a weekly basis.
In the every-three-week regimen, patients received paclitaxel
200 mg/m2 and carboplatin with AUC of six. The chemotherapy in
the weekly regimen consisted of paclitaxel 80 mg/m2 with carboplatin
AUC of two. The combination was given three out of four weeks.
During the fourth week, patients received trastuzumab alone.
Our target accrual is 92 patients and enrollment is nearly completed.
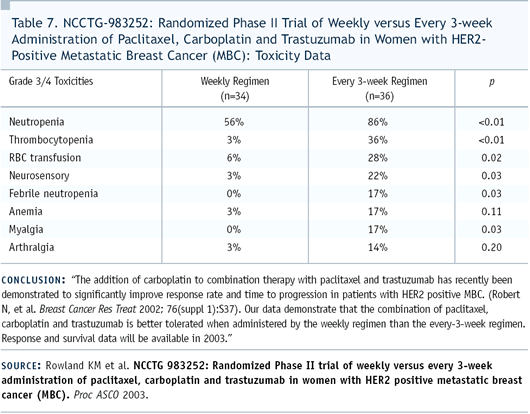
We submitted an abstract to ASCO, documenting a significant difference
in tolerability between the two regimens. Although the every-three-week
regimen was fairly well-tolerated, we saw myelosuppression, some
cases of febrile neutropenia, and some neuropathy.
However, when we looked at the tolerability of the weekly regimen,
it was remarkably different in that we essentially did not see
any myelosuppression or febrile neutropenia. And the difference
in myelosuppression was not only in the white blood cell count
but also with anemia. We also saw very few cases of neuropathy.
Our conclusion was that we would recommend the weekly regimen be
utilized in view of tolerability (Table 7).
The efficacy data on the first 70 patients is quite favorable.
This is a randomized Phase II study, so we have to be a little
bit careful. This is not really a randomized Phase III comparison
of one regimen versus another, but it is a multi-institutional
trial in which many of the patients were enrolled in community
settings, so the patients actually were not enrolled at the Mayo
Clinic.
Although the 95 percent confidence intervals of response rate
overlapped a little bit, it’s actually much better to give
the combination on a weekly schedule rather than every three weeks.
We are currently using the weekly regimen in patients, even outside
this trial. Based on the data from NCCTG-983252, weekly paclitaxel/carboplatin/trastuzumab
has a better therapeutic ratio than using the drugs once every
three weeks.
 |
| Case follow-up: |
 |
| • |
Patient treated with trastuzumab/carboplatin
and docetaxel (75 mg/m2) |
| • |
Very rapid clinical improvement; breast became
less swollen and painful |
| • |
After 3 cycles, no palpable abnormality in breast, ultrasound
with marked improvement, pulmonary nodules decreased in size |
| • |
Plan is for patient to complete 6 cycles of chemotherapy,
undergo breast irradiation, and continue trastuzumab |
 |
Select publications
|
