|

Objectives |
|
Eligibility |
- Metastatic breast cancer, previously untreated with chemotherapy
- HER2 2+ or 3+ measured by the Clinical Trial Assay (CTA)
- FISH testing for HER2 gene amplification
|
Schema |
ARM 1: Chemotherapy* q 3 weeks x 6
ARM 2: Chemotherapy* q 3 weeks x 6 plus trastuzumab q week |
*Chemotherapy = AC, if no prior adjuvant anthracycline, or paclitaxel if patient received prior adjuvant anthracycline-based chemotherapy
Trastuzumab was continued until disease progression. Upon disease progression, 66 percent of the women elected to receive trastuzumab alone or in combination with other therapies.
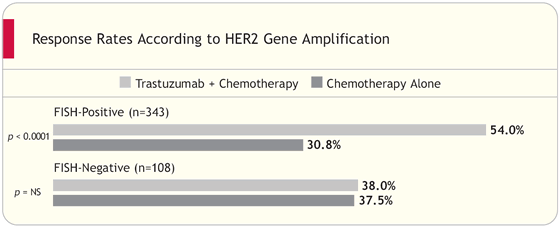
Authors’ Conclusions
“Patient selection based on HER2/neu amplification by FISH may predict improved clinical benefit from the addition of H [Herceptin®] to C [chemotherapy] compared to selection by IHC (2+/3+). This includes a substantial survival benefit. This data supports FISH testing for selecting patients for Herceptin therapy.”
| Research Leader Commentary |
In our previous studies, FISH was the most accurate method for assessing HER2 status, and commercially-available FDA-approved IHC assays were significantly less accurate. We collaborated to re-evaluate the tissue from women who had been enrolled in the trastuzumab pivotal trials.
We found somewhere between one out of four and one out of five women entered into those trials, based upon IHC, actually did not have HER2 gene amplification. In other words, there were false-positive results by IHC. When we analyzed the data, FISH was found to be a stronger predictor of response to trastuzumab.
Michael F Press MD, PhD |
Page 2 of 6
Previous | Next
|
|



