 |
Comparison of HER2 Assays |

Objectives |
|
Methods |
-
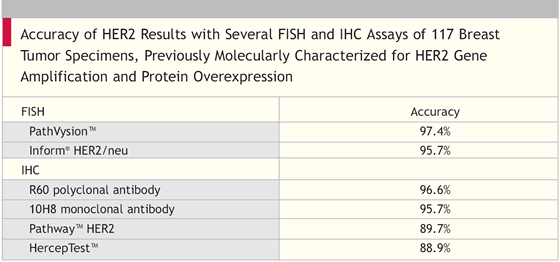
117 breast tumor specimens, previously molecularly characterized for HER2 gene amplification and protein overexpression, were evaluated for HER2 gene amplification by FISH with the PathVysionTM assay and Inform® HER2/neu assay.
-
The same specimens were also evaluated for HER2 protein overexpression by IHC with the HercepTestTM assay, PathwayTM HER2 assay, R60 polyclonal antibody and 10H8 monoclonal antibody.
|
Authors’ Conclusions
“Our findings demonstrated that the FISH assays have higher sensitivity and higher accuracy and more often correctly identify altered HER-2/neu status (amplification/overexpression) in previously molecularly characterized specimens than did the FDA-approved immunhistochemistry assays interpreted manually.”
| Research Leader Commentary |
This analysis actually compared several different reagents. Press and colleagues used a polyclonal antibody and compared it to some monoclonal antibodies, including 10H8 (not yet widely used and not FDA-approved), Ventana’s CB-11, and the Dako HercepTestTM. I caution that one can alter the sensitivity or specificity of any reagent, so a ranking that’s slightly (2% or 3%) better should be taken with a grain of salt. It sounds like the antibodies are pretty much in same ballpark.
Ann D Thor, MD |
Page 1 of 4
Next
|
|



