|

Objectives |
- To determine the quality of HER2 assays performed in laboratories
nationwide
|
Methods |
-
A central review of the first 104 cases entered into the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial was conducted.
-
Eligibility for NSABP-B-31 required a score of 3+ with the HercepTestTM [IHC assay], strong membrane staining (>33 percent of the tumor cells) with other IHC assays, or gene amplification with FISH.
- A central laboratory assayed the tumor blocks for these cases with both the HercepTestTM and the PathVysionTM FISH assay.
|
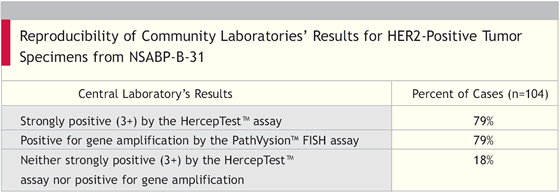
Concordance for Central Testing
There was good concordance between central testing for FISH and HercepTestTM, with agreement in 94 percent of the cases.
Authors’ Conclusions
“This brief communication provides a snapshot of the quality of HER2 assays nationwide. We found that an appreciable percentage of community-based assay results, which were used to establish the eligibility of patients to participate in NSABP-B-31, could not be confirmed when tested in a central facility.
“Our data suggest a need to improve quality control measures in laboratories that use IHC assays, including periodic testing for concordance with FISH…. Accordingly, the NSABP has amended eligibility criteria for B-31: only patients whose tumors score 3+ by IHC performed by NSABP-approved reference laboratories, or whose tumors demonstrate gene amplification by FISH from any laboratory, would be allowed entry.”
| Research Leader Commentary |
This was a wake-up call to clinicians in the community about how HER2 assays perform in the real world. Community laboratories don’t have the same performance when compared to the “gold standard” of commercial reference laboratories. Therefore, it is important to find out who is doing the HER2 testing. Good clinicians can also take other clinical variables into account to decide about retesting.
There is a good deal of evidence showing a correlation between the number of cases one analyzes per week with IHC and assay performance, and that’s where commercial laboratories win hands down. They do many more tests per week than a small hospital in rural North America. The bottom line is there is a learning curve with respect to reading IHC stains. To get to the top of the curve, you have to read a lot of them, and the only way to do that is to be in a in a big, busy center or in a commercial laboratory.
Mark Pegram, MD
There remains considerable controversy regarding the optimal method to routinely evaluate HER2 status. I won’t treat a patient with metastatic breast cancer until I have a FISH assay. In the June 2002 issue of the Journal of the National Cancer Institute, the NSABP and the Intergroup published their experiences with HER2 assessment, and it really cast doubt about our quality control for immunohistochemistry. Until the College of American Pathologists does something to iron out this problem of quality control, I continue to use FISH.
Charles Vogel, MD, FACP
I assume that the tumors with a 3+ score on immunohistochemistry (IHC) are truly HER2- positive, and we do not test them further. An IHC score of 3+ is pretty reliable, as long as it is done at a laboratory that performs a lot of assays. If a tumor has a 2+ score on IHC, we test with fluorescence in situ hybridization (FISH). Even in patients with an IHC score of 0 or 1+ and other features of excessively aggressive disease, we may also do a FISH test.
Both the Intergroup and the NSABP study discovered that smaller community hospitals were overscoring tumors as 3+. Close to 20 percent of the 3+ scores were downstaged when they were reviewed centrally. The Intergroup protocol has now been amended to require that the patients wait for final randomization until there is a central review of their HER2 status.
I think the same things apply to FISH testing. Since FISH testing already tends to be done at more centralized laboratories, we have not yet explored the quality control issues. I suspect there will be a proliferation of FISH testing, and the reagents will go out to all the community hospitals. Even though there is probably less room for interobserver variability, the same issues will apply. I hope as the FISH technology disseminates, people will do these quality control-type studies.
At some point, it may be possible that the only test that will be done is FISH. I believe it to be more accurate and less subject to interobserver variability. I think the cost should be downplayed if it is only a difference of $100 or $200. However, when trastuzumab is given incorrectly for several months, that involves many thousands of dollars. It behooves us all — even from a cost standpoint — to have the most accurate test up and running.
Debu Tripathy, MD |

Objectives |
|
Methods |
-
A central review of the first 240 cases enrolled after the protocol amendment was conducted.
-
Eligibility for NSABP-B-31 required a score of 3+ on IHC from an NSABP-approved laboratory or gene amplification with FISH from any laboratory.
- A central laboratory assayed the tumor blocks for these cases with the PathVysionTM FISH assay.
|
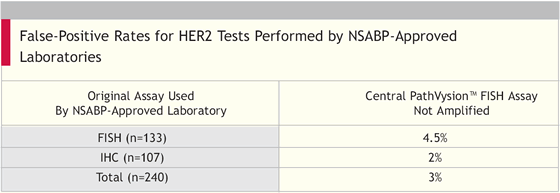
Authors’ Conclusions
“We believe that the quality assurance program resulted in a dramatic improvement in the reliability of HER2 testing by IHC. The false-positive rate, as defined by FISH, decreased from 21 percent to 2 percent (p = 0.003). The quality assurance program achieved the goal of reducing the false-positive rate below 10 percent, actually achieving a 3 percent overall false-positive rate.”
| Research Leader Commentary |
After laboratories underwent training from the NSABP and became certified, their accuracy went way up. Several things can be done to improve performance and reduce variability. One is to train the interpreter. Another is to have the laboratory be certified. It’s very important that laboratories participate voluntarily in these quality control programs and that they use controls with every assay.
Oncologists need to be more aware of which laboratory performs the tests and who interprets the results, because it can make a huge difference. Whether it’s a hospital-based laboratory or a reference laboratory, I think the oncologist should spend a lot of time getting to know their laboratories, which tests they’re using, and how they read the results and interpret oncology and pathology guidelines.
Ann D Thor, MD
The NSABP found the discordance rate to be much lower when experienced or certified laboratories for HER2 testing are used. This is really good for clinical care, because HER2 testing is not only being done for patients potentially eligible for clinical protocols, but also in general clinical practice.
Edith A Perez, MD |
Page 2 of 3
Previous | Next
|
|



