
 |
||||||||

| Tracks 1-23 | ||||||||||||||||||||||||||||||||||||||||||||||||
|
Tracks 1-4

![]() DR LOVE: If this patient had asked you what her risk of recurrence would
be without any systemic therapy, what would you have told her?
DR LOVE: If this patient had asked you what her risk of recurrence would
be without any systemic therapy, what would you have told her?
![]() DR LEYLAND-JONES: It is difficult to project for these cohorts with HER2-positive tumors that are smaller than one centimeter. Having said that,
pathology drives everything, and this case had one of the most aggressive
pathologies I have ever seen. I believe her chances of recurrence were
extremely high and that her tumor would have already seeded vascularly.
DR LEYLAND-JONES: It is difficult to project for these cohorts with HER2-positive tumors that are smaller than one centimeter. Having said that,
pathology drives everything, and this case had one of the most aggressive
pathologies I have ever seen. I believe her chances of recurrence were
extremely high and that her tumor would have already seeded vascularly.
![]() DR LOVE: Mark, if this women had asked, “What do you think is my best
choice for treatment?” what would you have said?
DR LOVE: Mark, if this women had asked, “What do you think is my best
choice for treatment?” what would you have said?
![]() DR PEGRAM: I probably would have recommended the combination of
endocrine therapy and trastuzumab. I would have put chemotherapy on the
table as an option but showed her my best guess of its absolute benefits.
DR PEGRAM: I probably would have recommended the combination of
endocrine therapy and trastuzumab. I would have put chemotherapy on the
table as an option but showed her my best guess of its absolute benefits.
It is important to point out absolute benefits rather than relative risk reductions from adjuvant chemotherapy to put it into a perspective that patients can understand. Then I would let the patient weigh in on the decision. I’m not going to tell a patient “yea” or “nay” when it comes to these gray areas.
![]() DR LOVE: Brian, what actually happened with this patient?
DR LOVE: Brian, what actually happened with this patient?
![]() DR LEYLAND-JONES: We ended up treating her with FEC followed by trastuzumab,
mainly because she was young and wanted to preserve her cardiac
function.
DR LEYLAND-JONES: We ended up treating her with FEC followed by trastuzumab,
mainly because she was young and wanted to preserve her cardiac
function.
Mark, what did you think about the TAnDEM data (Mackey 2006) evaluating trastuzumab with an aromatase inhibitor in metastatic disease?
![]() DR PEGRAM: Adding trastuzumab doubled the time to tumor progression.
This demonstrates a measurable treatment effect of the addition of trastuzumab
to an aromatase inhibitor in first-line metastatic breast cancer that’s HER2-positive and ER-expressing. That establishes precedent in my mind that those
types of combinations would probably have as much or more activity in the
adjuvant setting.
DR PEGRAM: Adding trastuzumab doubled the time to tumor progression.
This demonstrates a measurable treatment effect of the addition of trastuzumab
to an aromatase inhibitor in first-line metastatic breast cancer that’s HER2-positive and ER-expressing. That establishes precedent in my mind that those
types of combinations would probably have as much or more activity in the
adjuvant setting.
You could argue that the TAnDEM data set could be used as the basis for my recommendation, even though the results are modest and it’s in metastatic breast cancer (Mackey 2006; [3.1]).
![]() DR LEYLAND-JONES: I’m going to be “tongue in cheek” now and argue
against myself.
DR LEYLAND-JONES: I’m going to be “tongue in cheek” now and argue
against myself.
If you consider the control arm of the TAnDEM study with those who had centrally confirmed ER-positive tumors, the time to progression was approximately 3.8 months, whereas for a HER2-negative population it is on the order of 11 to 12 months, so there is a significantly reduced effect for hormone therapy in the HER2-positive population.
![]() DR PEGRAM: This is exactly as we predicted based on publications on this issue of HER2 and estrogen receptor cross talk in laboratory models, which
we first published in 1995 (Pietras 1995). HER2 would confer relative
endocrine resistance.
DR PEGRAM: This is exactly as we predicted based on publications on this issue of HER2 and estrogen receptor cross talk in laboratory models, which
we first published in 1995 (Pietras 1995). HER2 would confer relative
endocrine resistance.
![]() DR LEYLAND-JONES: One of the conclusions that José Baselga drew from
that study is that those patients probably need chemotherapy as opposed to
hormone therapy. So in the way that we’re administering it, the chemotherapy
and trastuzumab is the major treatment modality, and the hormone therapy is
the additive part of treatment.
DR LEYLAND-JONES: One of the conclusions that José Baselga drew from
that study is that those patients probably need chemotherapy as opposed to
hormone therapy. So in the way that we’re administering it, the chemotherapy
and trastuzumab is the major treatment modality, and the hormone therapy is
the additive part of treatment.
![]() DR PEGRAM: I believe the key to success of adjuvant therapy for this patient
will be the HER2-directed therapy. That treatment will have the greatest
impact in terms of proportional reduction and relative odds of recurrence.
DR PEGRAM: I believe the key to success of adjuvant therapy for this patient
will be the HER2-directed therapy. That treatment will have the greatest
impact in terms of proportional reduction and relative odds of recurrence.
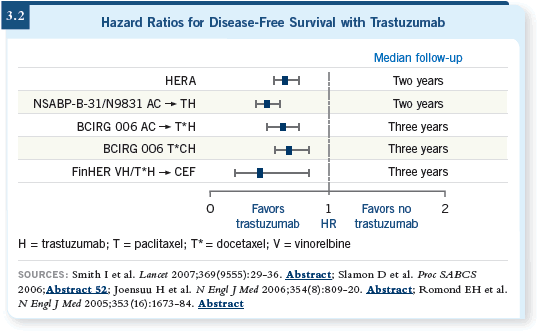
The hazard ratio across the board for all of the adjuvant trastuzumab trials ( Joensuu 2006; Piccart-Gebhart 2005; Romond 2005; Slamon 2006; Smith 2007) is approximately one half, and that’s above and beyond chemotherapy (3.2).
![]() DR LEYLAND-JONES: We’re dealing with an unknown here. Martine Piccart
made a beautiful presentation at the Istanbul meeting last October, in which
she was asking what we do with tumors smaller than one centimeter. She
showed this beautiful blue slide with two words written on it: “Nobody
knows.” So we are projecting here using the metastatic data from the
TAnDEM trial (Mackey 2006).
DR LEYLAND-JONES: We’re dealing with an unknown here. Martine Piccart
made a beautiful presentation at the Istanbul meeting last October, in which
she was asking what we do with tumors smaller than one centimeter. She
showed this beautiful blue slide with two words written on it: “Nobody
knows.” So we are projecting here using the metastatic data from the
TAnDEM trial (Mackey 2006).
Whether we did the right thing or not, I do not know, but if you do consider those metastatic data — trastuzumab alone in metastatic disease — you see the times to progression are between three to four months. With the chemotherapy and trastuzumab, the times to progression — whether it’s a taxane or vinorelbine — are 10 to 12 months.
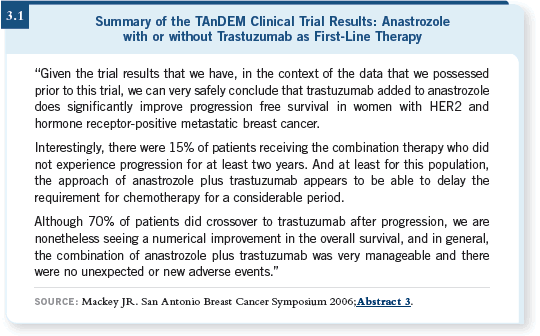
This is a lot of your synergy data, Mark. I would agree with you completely that the major benefit was from the trastuzumab. However, there would seem to be, if you project from metastatic data, distinct synergy from trastuzumab and the chemotherapy together. Now we are worried about administering trastuzumab alone in the adjuvant setting because we have no data for it.
Tracks 5-9

![]() DR PEGRAM: For this patient, I simulated as best I could what might be the
relative risk reduction with cytotoxic therapy and with endocrine therapy
alone and concluded that the single most important component of her adjuvant
therapy would arguably be trastuzumab. After evaluating simulations using
Adjuvant! Online and evaluating the utility of chemotherapy for these small
tumors with ER-positive disease, we decided that chemotherapy would not be
a good choice.
DR PEGRAM: For this patient, I simulated as best I could what might be the
relative risk reduction with cytotoxic therapy and with endocrine therapy
alone and concluded that the single most important component of her adjuvant
therapy would arguably be trastuzumab. After evaluating simulations using
Adjuvant! Online and evaluating the utility of chemotherapy for these small
tumors with ER-positive disease, we decided that chemotherapy would not be
a good choice.
For this woman, we used endocrine therapy in combination with trastuzumab. We used tamoxifen because she was 53 years old and early perimenopausal.
![]() DR LOVE: Are there any situations in which either of you would use ovarian
suppression and an aromatase inhibitor off protocol for a premenopausal patient
with HER2-positive disease?
DR LOVE: Are there any situations in which either of you would use ovarian
suppression and an aromatase inhibitor off protocol for a premenopausal patient
with HER2-positive disease?
![]() DR LEYLAND-JONES: We have used it once for a HER2-positive tumor in a
patient who had high-grade disease. In the vast majority of our cases, we’re
administering tamoxifen and ovarian suppression.
DR LEYLAND-JONES: We have used it once for a HER2-positive tumor in a
patient who had high-grade disease. In the vast majority of our cases, we’re
administering tamoxifen and ovarian suppression.
![]() DR PEGRAM: I must plead guilty and admit I have done it.
DR PEGRAM: I must plead guilty and admit I have done it.
For patients with 10 or more positive lymph nodes, what are you going to do? You want to maximize, biologically, the probability of response. I am impressed that, in adjuvant cohorts, aromatase inhibitors are somewhat better than tamoxifen for postmenopausal patients.
So if you simulate a postmenopausal state in a premenopausal woman, either biochemically or with surgical ovarian ablation, then you should be able to capture that same small but significant advantage of the aromatase inhibitors over tamoxifen, even in premenopausal patients, theoretically.
![]() DR LEYLAND-JONES: I agree with Mark, and I believe the combination of
ovarian ablation and aromatase inhibitors will be used increasingly, Neil.
DR LEYLAND-JONES: I agree with Mark, and I believe the combination of
ovarian ablation and aromatase inhibitors will be used increasingly, Neil.
![]() DR LOVE: Mark, we should point out that your openness to using trastuzumab
in the adjuvant setting without chemotherapy is not something that we
see in a lot of clinical investigators and oncologists in practice. Everybody says,
“Well, you want to at least stick a taxane in there.” In fairness, we should say
that your approach is a little unusual.
DR LOVE: Mark, we should point out that your openness to using trastuzumab
in the adjuvant setting without chemotherapy is not something that we
see in a lot of clinical investigators and oncologists in practice. Everybody says,
“Well, you want to at least stick a taxane in there.” In fairness, we should say
that your approach is a little unusual.
![]() DR PEGRAM: Sure, and I don’t suggest that everybody should jump to use this
approach. However, in terms of enthusiasm for the approach, a rising swell of
clinical investigators want to tackle this question in a clinical trial.
DR PEGRAM: Sure, and I don’t suggest that everybody should jump to use this
approach. However, in terms of enthusiasm for the approach, a rising swell of
clinical investigators want to tackle this question in a clinical trial.
Tracks 10-11

![]() DR PEGRAM: Obviously, patients like this woman were excluded from the
adjuvant trastuzumab studies, as they probably should have been. However, we
have to understand that her risk of recurrence and death is so high that if you
don’t maximize efficacy at this point, she will succumb to her disease.
DR PEGRAM: Obviously, patients like this woman were excluded from the
adjuvant trastuzumab studies, as they probably should have been. However, we
have to understand that her risk of recurrence and death is so high that if you
don’t maximize efficacy at this point, she will succumb to her disease.
I would still be inclined to consider a trastuzumab-based adjuvant scheme, pray that her heart will be okay and follow her closely. This comes up occasionally in metastatic breast cancer, too, and at some point the patient’s breast cancer will be as or more life threatening than the risk of cardiomyopathy associated with the trastuzumab. When that boundary is crossed, then trastuzumab becomes a therapeutic option.
Chemotherapy also has to be on the table in this case. I would try it, but I would bail out in the event of any toxicity. Weekly taxanes are particularly well tolerated. I would have to read the literature on hemodialysis and paclitaxel.
I don’t know what effect it has on the pharmacology of paclitaxel. Anthracyclines are off the table in this case because of the low ejection fraction.
![]() DR LEYLAND-JONES: Would you administer taxanes and trastuzumab concurrently?
DR LEYLAND-JONES: Would you administer taxanes and trastuzumab concurrently?
![]() DR PEGRAM: Yes, I would probably try weekly paclitaxel/trastuzumab.
DR PEGRAM: Yes, I would probably try weekly paclitaxel/trastuzumab.
![]() DR LOVE: What do we know about the cardiac safety of a taxane and trastuzumab,
without any prior anthracycline, compared to nothing?
DR LOVE: What do we know about the cardiac safety of a taxane and trastuzumab,
without any prior anthracycline, compared to nothing?
![]() DR PEGRAM: We have the data from several randomized clinical trials in
which paclitaxel/trastuzumab has been one of the treatment arms.
DR PEGRAM: We have the data from several randomized clinical trials in
which paclitaxel/trastuzumab has been one of the treatment arms.
The Robert study compared paclitaxel/trastuzumab to paclitaxel/carboplatin/ trastuzumab (Robert 2006). That’s in the metastatic setting, but at least we have the safety data. Paclitaxel/trastuzumab used alone has no real impact on ejection fraction.
We also have the pivotal trial data in metastatic disease (Slamon 2001). I will not say there was no impact on cardiac functioning. Anecdotes exist of depressed ejection fractions in patients who were treated even with single-agent trastuzumab. So you’re not going to say it has no impact, but the risk is low.
In the BCIRG 006 cohort — which did not receive paclitaxel but rather docetaxel/carboplatin and trastuzumab — the risk of clinical congestive heart failure or Grade III/IV cardiotoxicity was extremely low.
It’s disconcerting that this patient is starting out with known cardiac disease, but it’s more disconcerting that she has such a high risk of recurrence and will probably die from breast cancer if you don’t attempt adjuvant therapy in order to gain distant disease control.
![]() DR LOVE: Brian, how did you think this case through, and what did you do?
DR LOVE: Brian, how did you think this case through, and what did you do?
![]() DR LEYLAND-JONES: This was made easy for us, Neil, because she refused
to undergo any kind of intravenous or systemic therapy. She had a psychiatric
history, and the consensus of the group at tumor board was to treat her with
an aromatase inhibitor alone.
DR LEYLAND-JONES: This was made easy for us, Neil, because she refused
to undergo any kind of intravenous or systemic therapy. She had a psychiatric
history, and the consensus of the group at tumor board was to treat her with
an aromatase inhibitor alone.
I must admit that the risk of recurrence here is extremely high, and I would have leaned in Mark’s direction. I would probably have gone back to the cardiologist and asked whether, if we’d played around with the cardiac protective medications and an ACE inhibitor, we could have kicked the LVEF up a bit more.
The weekly paclitaxel and trastuzumab would probably be the optimal therapeutic index. The other option would have been an aromatase inhibitor with trastuzumab, as we discussed in the previous case.
Tracks 12-14

![]() DR LEYLAND-JONES: Generally, we administer neoadjuvant therapy, with
something like the Buzdar regimen by which we administer all the chemotherapy
up front (Buzdar 2005). Our own leaning is more toward administering
something like AC
DR LEYLAND-JONES: Generally, we administer neoadjuvant therapy, with
something like the Buzdar regimen by which we administer all the chemotherapy
up front (Buzdar 2005). Our own leaning is more toward administering
something like AC![]() TH or FEC
TH or FEC![]() TH for four cycles of each.
TH for four cycles of each.
![]() DR LOVE: Are you administering the trastuzumab during the anthracycline,
as Buzdar did?
DR LOVE: Are you administering the trastuzumab during the anthracycline,
as Buzdar did?
![]() DR LEYLAND-JONES: We’re not at this moment, but my understanding is that
a publication from Buzdar will appear fairly soon, in which the safety of that
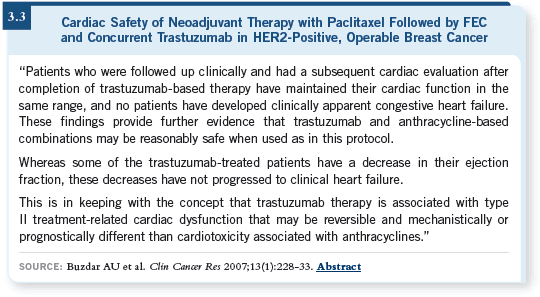
regimen is confirmed (Buzdar 2007; [3.3]).
DR LEYLAND-JONES: We’re not at this moment, but my understanding is that
a publication from Buzdar will appear fairly soon, in which the safety of that
regimen is confirmed (Buzdar 2007; [3.3]).
![]() DR LOVE: For patients who have residual tumor and positive nodes, what do
you do postoperatively?
DR LOVE: For patients who have residual tumor and positive nodes, what do
you do postoperatively?
![]() DR LEYLAND-JONES: We continue the trastuzumab for a full year, without
any other type of chemotherapy.
DR LEYLAND-JONES: We continue the trastuzumab for a full year, without
any other type of chemotherapy.
![]() DR LOVE: Mark, what did you do in this situation?
DR LOVE: Mark, what did you do in this situation?
![]() DR PEGRAM: We administered docetaxel/carboplatin and trastuzumab. This
was, at least in part, based on a collaboration we’ve had with Judith Hurley in
Miami, who published a paper in the JCO last year showing that in a cohort of
similar patients with large primary tumors, she obtained a respectable pathologic
complete response rate with TCH (Hurley 2006).
DR PEGRAM: We administered docetaxel/carboplatin and trastuzumab. This
was, at least in part, based on a collaboration we’ve had with Judith Hurley in
Miami, who published a paper in the JCO last year showing that in a cohort of
similar patients with large primary tumors, she obtained a respectable pathologic
complete response rate with TCH (Hurley 2006).
The pathologic complete response rate was approximately 26 percent, which compares favorably to any other cytotoxic induction for locally advanced disease.
Considering she achieved that in a cohort of patients with a massive median tumor size, we thought that was impressive and had no hesitation recommending the regimen off protocol to this patient.
![]() DR LOVE: What happened when she was treated?
DR LOVE: What happened when she was treated?
![]() DR PEGRAM: She had a fantastic clinical response. She literally had no
palpable disease by the time she finished her cytotoxic induction regimen, but
on histopathology she still had multiple residual, small areas of invasive disease
in the area of the primary and multiple, small metastases by H&E stain in the
axillary lymph nodes.
DR PEGRAM: She had a fantastic clinical response. She literally had no
palpable disease by the time she finished her cytotoxic induction regimen, but
on histopathology she still had multiple residual, small areas of invasive disease
in the area of the primary and multiple, small metastases by H&E stain in the
axillary lymph nodes.
![]() DR LOVE: Postoperatively, she received trastuzumab alone?
DR LOVE: Postoperatively, she received trastuzumab alone?
![]() DR PEGRAM: She received trastuzumab with concurrent chest wall radiation
therapy. You’re always uncertain with these neoadjuvant cases that don’t
show pathologic complete responses whether or not to administer additional
cytotoxic therapy later on.
DR PEGRAM: She received trastuzumab with concurrent chest wall radiation
therapy. You’re always uncertain with these neoadjuvant cases that don’t
show pathologic complete responses whether or not to administer additional
cytotoxic therapy later on.
In the absence of data, it’s difficult to recommend that, although emotionally we all want to believe it will help. We wound up not administering any additional cytotoxic therapy.
Tracks 15-21
![]() DR PEGRAM: This is first-line metastatic breast cancer, so I’m going to
consider trastuzumab-based regimens because the patient’s cardiac status is okay.
DR PEGRAM: This is first-line metastatic breast cancer, so I’m going to
consider trastuzumab-based regimens because the patient’s cardiac status is okay.
She would be a candidate for a cytotoxic therapy in combination with trastuzumab. If she’s young and otherwise healthy, she could probably even consider combination cytotoxics along with the trastuzumab, such as TCH, but I don’t feel strongly with metastatic disease about combinations versus single agents or about which agents are used, as long as it’s trastuzumab based.
I suspect that a 31-year-old woman will likely see multiple cytotoxics over time. It is just a matter of trying to maximize the probability of obtaining control of her disease up front and then palliation thereafter. Of course, this would be used in combination with a bisphosphonate for her bone metastases.
![]() DR LOVE: Brian, how did you approach this patient?
DR LOVE: Brian, how did you approach this patient?
![]() DR LEYLAND-JONES: She was treated with carboplatin/paclitaxel and trastuzumab
with pamidronate.
DR LEYLAND-JONES: She was treated with carboplatin/paclitaxel and trastuzumab
with pamidronate.
![]() DR LOVE: What do you think your second-line therapy will be?
DR LOVE: What do you think your second-line therapy will be?
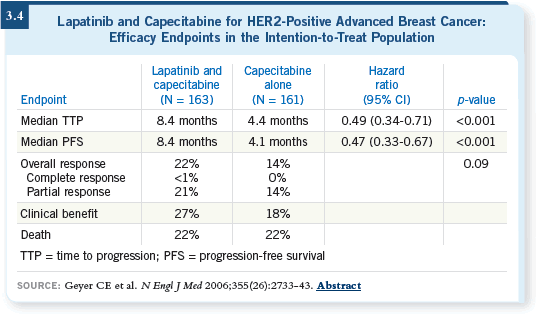
![]() DR LEYLAND-JONES: We will probably follow the Chuck Geyer data (Geyer
2006; [3.4]) and recommend capecitabine/lapatinib.
DR LEYLAND-JONES: We will probably follow the Chuck Geyer data (Geyer
2006; [3.4]) and recommend capecitabine/lapatinib.
![]() DR LOVE: How would you decide between that and keeping the trastuzumab
going and adding another chemotherapeutic agent?
DR LOVE: How would you decide between that and keeping the trastuzumab
going and adding another chemotherapeutic agent?
![]() DR LEYLAND-JONES: A big controversy existed about continuing trastuzumab
on progression. It seemed people would say, “Well, if the patient had a good,
prolonged response to first-line therapy, I might be more likely to continue
the trastuzumab.” Will that now go totally out the window, and will people
simply go to second-line lapatinib?
DR LEYLAND-JONES: A big controversy existed about continuing trastuzumab
on progression. It seemed people would say, “Well, if the patient had a good,
prolonged response to first-line therapy, I might be more likely to continue
the trastuzumab.” Will that now go totally out the window, and will people
simply go to second-line lapatinib?
![]() DR PEGRAM: A strong sentiment will probably emerge to change classes of
inhibitors. The lessons learned from other targeted therapy approaches — the
estrogen receptor — are that by changing the strategy of therapeutic targeting,
you might capture additional responses, albeit with perhaps somewhat lower
frequency and not as long a duration, but nevertheless resulting in tangible
clinical benefit.
DR PEGRAM: A strong sentiment will probably emerge to change classes of
inhibitors. The lessons learned from other targeted therapy approaches — the
estrogen receptor — are that by changing the strategy of therapeutic targeting,
you might capture additional responses, albeit with perhaps somewhat lower
frequency and not as long a duration, but nevertheless resulting in tangible
clinical benefit.
This issue of trastuzumab duration in the metastatic setting has never been put to rest in a randomized clinical trial, which is unfortunate because once the tyrosine kinase inhibitors are available in the community, that question will probably become impossible to address.
Tracks 22-23

![]() DR LOVE: How did you manage this patient, Mark?
DR LOVE: How did you manage this patient, Mark?
![]() DR PEGRAM: We used weekly low-dose paclitaxel/carboplatin/trastuzumab,
and she did well for a number of months but eventually had disease progression.
She was still fit and without symptoms. Her blood counts were fine,
and she had no problems in terms of ejection fraction. Her progression was
detected initially with a rise in tumor marker values. The CA27-29 was
doubling and she had a new hepatic metastasis, which again was not associated
with any biochemical liver dysfunction.
DR PEGRAM: We used weekly low-dose paclitaxel/carboplatin/trastuzumab,
and she did well for a number of months but eventually had disease progression.
She was still fit and without symptoms. Her blood counts were fine,
and she had no problems in terms of ejection fraction. Her progression was
detected initially with a rise in tumor marker values. The CA27-29 was
doubling and she had a new hepatic metastasis, which again was not associated
with any biochemical liver dysfunction.
![]() DR LOVE: Brian, what would you be thinking at this point?
DR LOVE: Brian, what would you be thinking at this point?
![]() DR LEYLAND-JONES: Most of my colleagues would use either vinorelbine and
trastuzumab or capecitabine and trastuzumab at this point, although without
any question, soon we will be moving on to capecitabine/lapatinib.
DR LEYLAND-JONES: Most of my colleagues would use either vinorelbine and
trastuzumab or capecitabine and trastuzumab at this point, although without
any question, soon we will be moving on to capecitabine/lapatinib.
![]() DR LOVE: What did you do, Mark?
DR LOVE: What did you do, Mark?
![]() DR PEGRAM: She received vinorelbine and continued trastuzumab in the
absence of supporting data, but that’s what we elected to do because she had
had a several-month time to progression while on the weekly low-dose TCH
and had no contraindications to continuation of the trastuzumab at this point.
DR PEGRAM: She received vinorelbine and continued trastuzumab in the
absence of supporting data, but that’s what we elected to do because she had
had a several-month time to progression while on the weekly low-dose TCH
and had no contraindications to continuation of the trastuzumab at this point.
She was on that for only about two to three months and then she once again experienced disease progression, indicated by a rise in tumor markers. That prompted reimaging studies, and disease progression was found in the lung and the liver, with new lesions and a substantial increase in the size of her existing lesions.
Surprisingly, she was still asymptomatic. She had a mild transaminase elevation, but it was less than 1.5 times the upper limit of normal — not too severe.
She developed complications with her port, with an infection followed by a thrombosis. Ultimately, the port had to come out, so we did not have great IV access. The question at that time was whether to continue intravenous therapy with further trastuzumab-based salvage chemotherapy or not. She chose not to receive further trastuzumab, and we treated her with single-agent capecitabine. Her disease was stable on that for a period and then subsequently progressed.